Abstract
The attention system can be divided into alerting, orienting, and executive control networks. The efficiency and independence of attention networks have been widely tested with the attention network test (ANT) and its revised versions. However, many studies have failed to find effects of attention network scores (ANSs) and inter-network relationships (INRs). Moreover, the low reliability of ANSs can not meet the demands of theoretical and empirical investigations. Two methodological factors (the inter-trial influence in the event-related design and the inter-network interference in orthogonal contrast) may be responsible for the unreliability of ANT. In this study, we combined the mixed design and non-orthogonal method to explore ANSs and directional INRs. With a small number of trials, we obtained reliable and independent ANSs (split-half reliability of alerting: 0.684; orienting: 0.588; and executive control: 0.616), suggesting an individual and specific attention system. Furthermore, mutual inhibition was observed when two networks were operated simultaneously, indicating a differentiated but integrated attention system. Overall, the reliable and individual specific ANSs and mutually inhibited INRs provide novel insight into the understanding of the developmental, physiological and pathological mechanisms of attention networks, and can benefit future experimental and clinical investigations of attention using ANT.
Recent cognitive and neuropsychological research highlighted the heterogeneity of attention system1. In this framework, Posner and Petersen2 divided the attention system into three networks that carry distinct functional demands. The alerting (A) system initiates and sustains a readiness in reaction to upcoming stimuli; the orienting (O) component mediates the selection of relevant information for processing; and the executive control (E) network is involved in error monitoring and conflict resolving3,4. The efficiency and independence of attention networks were widely measured with the attention network test (ANT) which was devised by Fan and colleagues5.
Using the ANT and its revised versions6,7,8, the attention network scores (ANSs) and inter-network relationships (INRs) have been measured and applied in a wide range of investigations such as development, psychiatric disorders, neuroimaging, and genetics3,4,9. However, the low reliability (split-half reliability: 0.14–0.71; test-retest reliability: 0.35–0.80) of ANSs may decrease statistical power, underestimate the strength of true inter-network correlation, and restrict the application of ANT in developmental and clinical studies10,11. In addition, the ANSs and INRs are undetected in a large proportion of studies10,12. Considering the widely usage of ANT in recent investigations about developmental differences, individual differences, and deficits in special populations3,13, improving the reliability of ANSs and illuminating the pattern of INRs are important and urgent tasks.
There are at least two methodological reasons that might be responsible for the instability of ANT. First, in the event-related design, the inter-network interplay would influence the efficiency of each network, because the present attention operation strongly depends on near past experience13,14. Allocating different networks into separate blocks/runs may avoid inter-network impact from past trials15. Thus, we revised the ANT with a mixed block/event-related design. In the mixed design, ANSs and INRs were tested in separate runs to avoid the influence from other components as much as possible when measuring one network or interaction. Second, orthogonal contrasts used in the ANT are susceptible to INRs10. Although the non-orthogonal method has been used to calculate ANSs12,13, these results were still affected by INRs in the event-related design. We suggested that the combination of non-orthogonal method and mixed design is more reasonable to accurately measure the ANSs.
With the mixed design, we allocated the measurement of ANSs and interactions into six separated blocks: three effect blocks were used to test three ANSs, respectively; three interaction blocks were used to examine pair-wise INRs. Specifically, spatial targets were excluded when measuring A and E to avoid the influence from O; cues were canceled when testing O and E to exclude the alerting effect; incongruent targets were eliminated when examining A and O to avoid the conflict effect. Two significant modifications should be noted. First, all effects measured here were stimulus-driven. The exogenous attention and endogenous attention have different time courses and neural mechanisms16,17. They are involved in previous measurements of ANSs and INRs to varying degrees, bringing difficulties in explaining ANSs and INRs. We endeavored to avoid the confounding of exogenous and endogenous mechanisms from informative spatial cue18 and varying cue-target intervals17 by eliminating spatial cue and using a constant cue-target interval of 300 ms16. Second, the effects of A and O were separated by removing cues when testing O rather than using a cross-modality operation7, because attention has been demonstrated to be modality dependent19,20. Furthermore, in the interaction version of ANT, both alerting tone and spatial cue may enhance phasic alerting12. To avoid inter-network interference and get more accurate measurements of ANSs and INRs, we allocated different ANSs and INRs into separate blocks/runs with a mixed design.
The non-orthogonal method was adopted to further avoid inter-network interference. Because of the interplay among diverse cue and target conditions, ANSs obtained by orthogonal and non-orthogonal methods were significantly different12,13. These researchers, therefore, suggested that the non-orthogonal method is better than the orthogonal method to get single measurements of ANSs. In the current study, nine equations were utilized to calculate ANSs and directional INRs. Combined with mixed design, this non-orthogonal method would reduce within-subject variability by avoiding inter-network interferences, thus obtaining more reliable and unsullied ANSs and directional INRs.
By separating the measurements of ANSs and inter-network interactions, we aimed to obtain more reliable and accurate ANSs and INRs. This endeavor would benefit investigations about the psychological and physiological mechanisms of attention and the application of ANT.
Results
Table 1 shows the RT and accuracy of each condition. Because the ceiling effect was evident, values of accuracy were not used for further analysis. Since RTs were not normally distributed, the median RT per condition was used for computing ANSs and directional INRs21. We replaced RTs with the median for trials with error responded (0.97%) or with the RT larger than 3 standard deviations (1.17%) in each cue-target condition in each subject. We replaced these data due to two reasons: 1) there are only 24 pairs of trial for each ANS which is much fewer than previous studies (e.g., 72 or 96 pairs of trial in the original version of ANT); 2) the analysis of split-half reliability requires equal numbers of trial for two halves.
Table 1. The reaction time and accuracy per condition.
| Effect | Condition | Reaction time | Accuracy |
|---|---|---|---|
| A | NC | 479.063 (57.942) | 0.998 (0.009) |
| CC | 434.750 (52.614) | 0.992 (0.016) | |
| O | CT | 480.744 (58.559) | 0.997 (0.011) |
| ST | 544.421 (69.706) | 0.993 (0.020) | |
| E | CO | 489.909 (55.435) | 0.996 (0.012) |
| IN | 550.142 (61.836) | 0.991 (0.020) | |
| A × O | NC_CT | 488.267 (60.470) | 0.996 (0.012) |
| NC_ST | 539.892 (65.214) | 0.992 (0.021) | |
| CC_CT | 441.097 (54.799) | 0.992 (0.019) | |
| CC_ST | 514.653 (63.084) | 0.988 (0.025) | |
| A × E | NC_CO | 503.330 (64.014) | 0.999 (0.006) |
| NC_IN | 555.432 (74.988) | 0.988 (0.023) | |
| CC_CO | 458.205 (57.942) | 0.999 (0.006) | |
| CC_IN | 531.699 (76.078) | 0.975 (0.041) | |
| O × E | CT_CO | 527.011 (65.145) | 0.998 (0.009) |
| CT_IN | 590.483 (73.913) | 0.988 (0.025) | |
| ST_CO | 612.632 (67.302) | 0.994 (0.014) | |
| ST_IN | 723.625 (83.658) | 0.948 (0.058) |
Note. Reaction time is in milliseconds. Standard deviations are in parenthesis. CC = central cue; NC = no cue; CT = central target; ST = spatial target; CO = congruent target; IN = incongruent target; A: alerting effect; O: orienting effect; E: executive control effect.
ANSs and directional INRs
As shown in Table 2 and Fig. 1, all ANSs and directional INRs were remarkably different from zero. For ANSs, obvious decrease in RTs could be seen on central cue condition than on no cue condition; while RTs were significantly increased on spatial target and incongruent target conditions than on central target and congruent target conditions, respectively. As shown in Fig. 1B, the O and E impaired the efficiency of A, indicating a weakened alert on spatial target and incongruent target conditions. The A enhanced the efficiencies of O and E, indicating slowed orienting and weakened executive control capacity in cued trials. The effects of O and E mutually enhanced, indicating slowed orienting on the incongruent target condition and impaired executive control capacity on the spatial target condition. These directional INRs revealed remarkable inter-network inhibition in interaction blocks, suggesting that the non-orthogonal method and mixed design were necessary to avoid inter-trial and inter-network interferences in measuring ANSs.
Table 2. Attention network scores and relationships between attention networks.
| Effect | Value (M±SD) | t(43) | P* |
|---|---|---|---|
| A | −0.091±0.049 | −12.346 | <0.0001 |
| O | 0.133±0.064 | 13.794 | <0.0001 |
| E | 0.125±0.063 | 13.113 | <0.0001 |
| A→O | 0.650±1.232 | 3.501 | 0.0011 |
| O→A | −0.545±0.841 | −4.300 | <0.0001 |
| A→E | 0.596±1.274 | 3.104 | 0.0034 |
| E→A | −0.505±0.772 | −4.340 | <0.0001 |
| O→E | 1.794±3.005 | 3.961 | 0.0003 |
| E→O | 0.804±0.885 | 6.027 | <0.0001 |
*Two-tailed. M: mean; SD: standard deviation; X→Y: the influence from X to Y.
Figure 1.
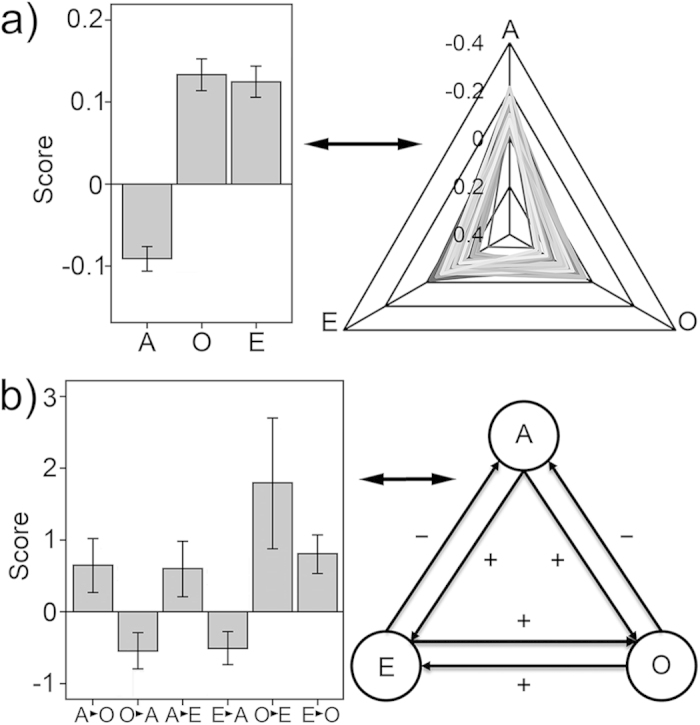
The attention network scores (ANSs) and directional inter-network relationships (INRs). (A) The mean ANSs (left) and their profiles on each subject (right). The A benefited RT, whereas O and E prolonged RT. Each person has a specific profile of A-O-E. (B) The mean INRs (left) and a simple framework of INRs (right). Both O and E reduced the effect of A. A enhanced effects of O and E. The effects of O and E were mutually promoted. X→Y: the influence from X to Y. Positive value represented the increase in RT, while negative value expressed the decrease in RT. Error bar shows the 95% confidence interval.
Inter-network interactions
As shown in Table 3, both main effects and interactions for three pairs of network were extremely significant, indicating strong coordination between networks when the cognitive system operates more than one network. Visualized inter-network interactions could be seen in Fig. 2. Post hoc analysis was not performed because the directional relationship analysis had described INRs in detail.
Table 3. The results of main effects and inter-network interactions in interaction blocks.
| Block | Effect | F(1, 43) | P |
|---|---|---|---|
| A × O | A | 82.783 | <0.0001 |
| O | 323.663 | <0.0001 | |
| Interaction | 22.496 | <0.0001 | |
| A × E | A | 115.983 | <0.0001 |
| E | 219.810 | <0.0001 | |
| Interaction | 26.800 | <0.0001 | |
| O × E | O | 1083.625 | <0.0001 |
| E | 231.921 | <0.0001 | |
| Interaction | 47.813 | <0.0001 |
Figure 2.
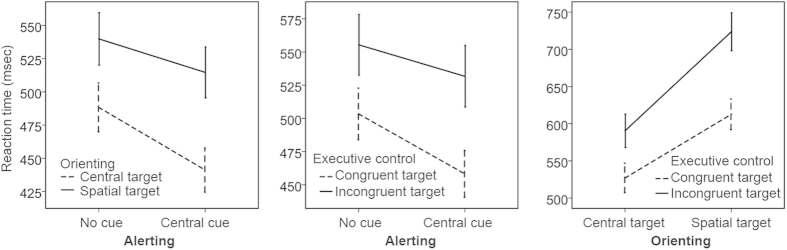
Inter-network interactions. Pairwise interactions were measured in separate blocks. All interactions of alerting × orienting, alerting × executive control, and orienting × executive control were significant. Specific inter-network relationships were shown in Fig. 1. Error bar shows the 95% confidence interval.
Inter-network correlations
For ANSs, Pearson correlation analysis showed a weak negative correlation between A and O: r = –0.310, P = 0.040. However, this correlation could not pass Bonferroni adjustment22. The correlations between A and E (r = 0.174, P = 0.257) and between O and E (r = 0.045, P = 0.772) were far from significance. These results revealed that the three networks are mutually independent.
For directional INRs, striking negative correlations between A→O and O→A (r = –0.411, P = 0.006) and between A→E and E→A (r =–0.348, P = 0.020), and positive correlation between O→E and E→O (r = 0.605, P < 0.0001) were observed (Fig. 3). Of note, the negative correlation was due to the negative value of A. These significant correlations indicated that the networks were mutually inhibited when two networks were operated simultaneously.
Figure 3.
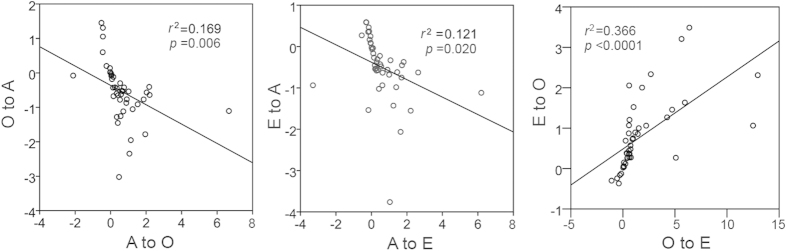
The correlations between directional inter-network relationships. If one network exerts more influence on another one, the reverse impact is increased simultaneously.
Reliability
The split-half reliabilities of A (0.684), O (0.588), and E (0.616) were all over 0.5. Considering that the split-half reliabilities of A (0.14–0.27) and O (0.26–0.38) were much lower than 0.5 in most studies10, the high reliabilities of three networks in the current study, therefore, have positive theoretical and clinical values.
Discussion
The reliability of ANSs and independency of networks suffered a long-term debate since Posner and Peterson2 put forward the three networks of attention system. Combined the non-orthogonal method with mixed design, we observed significant ANSs and INRs, indicating a differentiated but integrated attention system. Although with a few trials, we got very high split-half reliabilities of ANSs, suggesting that our method has a strong potential in measuring ANSs in developmental and clinical investigations.
The ANSs are significant and mutually independent. The lack of correlations among ANSs indicates that each person has a specific profile of A-O-E (Fig. 1A, right panel). In other words, a person with high O efficiency does not necessarily have high E efficiency, indicating separate mechanisms for three networks. Previous studies have demonstrated that the differentiation of three attention networks takes place in early ages. Specifically, the O matures at about 4 years1; the E gradually differentiates from O and matures during puberty23; the A gradually develops and matures during adolescence24. Essentially, the three networks are driven by different genes9 and neurotransmitters3,25. These genetic, developmental, and physiological factors may cooperate to create a specific profile of A-O-E on each person.
The independence and between subjects variability of ANSs are also supported by selective deficit in a variety of diseases. For example, specific E deficits have been observed in individuals with borderline personality disorder26 and posttraumatic stress disorder27. A specific O deficit has been reported in individuals with concussion28. A specific A deficit29 or both O and E deficits30 have been reported in different schizophrenia groups. The selective impairment of one or more networks indicates that the profile of A-O-E could serve as a psychological marker of a wide range of diseases31. However, these reports of selective impairments rely on the independence and reliability of ANSs10. The high reliability and remarkably independent ANSs in the present study suggested that combining non-orthogonal contrast with mixed design can provide a better measurement of the profile of A-O-E than previous studies and thus can benefit future theoretical and clinical studies.
The reliability of ANSs is particularly important given the widely application of ANT in developmental and clinical studies. Low reliability of A and O in previous studies not only fails to meet the demand of clinical investigations but also decreases statistical power and underestimates true inter-network correlations10,11. We suggest that the reliability of ANSs is constrained by at least three factors except for methodological factors announced in the Introduction section. First, the infra-slow fluctuations of RT are associated with significant intra-individual variation32,33,34, resulting in low reliability11. Second, more trials may increase reliability11 but induce fatigue effect that prolongs and increases variability of RTs35. Third, the fluctuations of arousal may increase the variability of observed ANSs on the one hand, because the ANSs are obtained by contrasting different effects with the baseline state; and the true ANSs on the other hand, because the arousal interacts and endogenously contributes to all three networks36. Therefore, reducing inter-trial variability or bringing it into statistical analysis is necessary to improve the reliability of ANSs.
The interaction between A and O has been reported by some researchers7,37 but not by others5,38. Those studies reporting a speeded-up orienting of attention under conditions of high alerting have proposed that the influence from A to O occurs only within a short (<500 ms) time course and peaks at about 150 ms7,17. They hypothesized that the A benefits the O7, 37, but provided no evidence and assumption on the influence from O to A. So far, there is not a theoretical framework about the interplay between A and O.
Beyond the unidirectional influence from A to O, we observed a larger effect of O when there was an alerting cue and a smaller effect of A when spatial target was presented. With a close inspection, we found that the alerting cue improved the respond to central target more than to spatial target, whereas the spatial target increased RTs to cued trials more than to un-cued trials. Therefore, the influence from A to O in the present study is opposite to previous findings. This discrepancy can be coordinated by a cue-target consistency effect. The consistent cue-target condition improves not only the phasic alerting but also the target processing at the cued location39,40, supporting the interaction between A and O. For the influence from A to O, when a spatial cue is presented as in previous studies, the processing of the target at that location is improved than at other locations. Reversely, if an alerting signal cues a central position, the processing of the target at this location is improved. For the influence from O to A, if the target appears at the same location as the cue, the processing is improved; if it appears at a different location from the cue, the processing is impaired. It’s worth noting that, in previous studies, the cue validity and cue-target interval may also contribute to the interaction between A and O by modulating both A and O via expectancy and time variability41. As a promising theoretical framework about the relationship between A and O, the cue-target consistency effect should be verified by manipulating cue validity and cue-target interval in future studies with behavioral and neuroimaging techniques25.
There are several opinions on the interaction between alerting and executive control networks. First, Callejas et al.7 proposed that alertness directly inhibits executive control by impeding the function of anterior cingulate cortex. Second, Böckler et al.42 and Fischer et al.43,44 suggested that alerting cues amplify automatic response activation related to both the relevant and irrelevant task dimensions, thus enhancing the conflict effect. Third, McConnell and Shore13 suggested that alerting cues encourage diffusing attention between two possible target locations. The uncertainty of target location in the ANT disturbs the attention in a focused state, making it harder to ignore distracting stimuli. Fourth, Fan et al.5 assumed that the alerting condition reduced RTs, thus providing less time to process conflict. Fifth, Weinbach and Henik45,46 demonstrated that alerting cues expand the attention scope and create a bias to global processing, thus increasing the disturbance of distracting stimuli. These views highlighted the influence from A to E, whereas the resource competition explanation focused on the bidirectional relationship between A and E6. Accordingly, the A and E may compete resources from their shared brain regions, e.g., the anterior cingulate cortex and fronto-parietal network.
By eliminating spatial targets, we observed enlarged conflict effect in the alerting condition as well as reduced alerting effect in the incongruent condition. These results are consistent with the resource competition explanation6, and can be partially explained by other perspectives except for McConnell and Shore’s13 target uncertainty hypothesis. It’s worth noting that the resource competition hypothesis can also explain the mutual inhibition between A and O, and between O and E. Given the complex and flexible interaction between A and E, more factors should be considered in future investigations such as the spatial pattern of targets46, dopamine level47, task difficulty36 and arousal48. We suggest that investigations on bidirectional relationship between A and E are essential to illuminate the mechanisms of alerting and executive control systems.
There are different assumptions about the influence from O to E in previous studies5,7,13. Fan et al.5 explained it from the perspective of attention resource that prior orienting of attention reduced RTs, resulting in insufficient time to process conflict. Callejas et al.7 suggested that the prior orienting of attention to the target location could help subjects to concentrate on that area and ignore the incongruent flankers. McConnell and Shore13 assumed that orienting to another location induced the global processing strategy, leading to a difficult in ignoring flankers. The confliction among these hypotheses lies in how subjects use attention resource and focusing strategy when orienting to a new position.
In this study, RTs of O were longer in incongruent condition than in congruent condition, while conflict effect was larger in spatial target condition compared with the central target condition. When O and E occur simultaneously, the resource competition hypothesis predicts bidirectional impairment between them, while the focusing strategy hypothesis anticipates an inhibition from O to E and a promotion from E to O because both E and O induce a global processing strategy. Therefore, the current results are consistent with the resource competition perspective rather than the focusing strategy hypothesis.
Convergent evidence showed that the attention system is a differentiated but integrated system. First, there is no consensus on the correlations among ANSs5,10,13,49, indicating a large inter-individual variability of the A-O-E profile. With high reliability of ANSs, the inter-network correlation is more properly estimated in the current study than in previous studies with low reliability of ANSs10,11. The lack of inter-network correlation we observed in a high homogeneous sample (college students), therefore, strongly propose a large inter-individual variability of the A-O-E profile. Many factors such as gene, development, physiology, and the state during test may be responsible for the particular A-O-E profile of each participant9,23,36,41,47.
Second, as an integrated system, attention resources would be allocated flexibly among networks. The resource competition hypothesis can explain the current findings as well as results in previous studies5,6. Meanwhile, inter-network cooperation is essential to produce a more adaptive behavior7. This requires the attention system to balance the roles of three networks dynamically37,41 and use various strategies such as global/local processing13,45,46 and multisensory integration7,40,49.
The differentiated networks and inter-network integration of attention are supported by different neurotransmitters and partially overlapped brain regions3,4,17. We also show that the networks are independent when they are separately measured, but mutually impaired when they are operated simultaneously. The differentiation and bidirectional impact of attention networks are of importance for our understanding of various A-O-E profiles in developmental and clinical investigations. For instance, if the E is differentiated from O23, can we expect stronger influence from O to E than from E to O in children before three years old compared with elder children and juveniles? In addition, if individuals with borderline personality disorder have a specific E deficit26, can we anticipate alterations of directional influence between E and A and between E and O? Our method may provide an effective solution to these developmental and clinical mysteries. Furthermore, reliable measurements of ANSs and directional INRs in a simple context are vitally important in developmental and clinical studies.
Combining non-orthogonal method with mixed design, we obtained independent ANSs with high reliability and remarkable bidirectional INRs. However, there are several limitations. First, the attention system is too complex to be measured by a single task. For example, several dichotomies have been highlighted in recent studies such as exogenous/endogenous attention17, tonic/phasic alerting46, top-down/bottom-up control14, and spatial/objective (non-spatial) attention46. Although the ANT tests a basic model of attention, complex relationships among more subcomponents should be examined in detail with reasonable reliability. Second, the cue-target interval in the present study is constant, eliminating the interference from temporal expectancy25,41. However, the inter-network relationship has been demonstrated to be time and probability dependent37,41. Cue validity, inter- and intra-trial possibilities, and more cue-target intervals are warranted in future studies to explore complex INRs. Third, our subjects were all college students with high homogeneity. This may not well reflect between-subject variability and the development and aging of attention system. A large sample size involves children, adults and old people may help us to dissociate between- and within-subject variability. Last, variables (e.g., sleep hours, time of testing, and drug consumption) who influence attention state should be recorded in future studies.
Conclusions
Compared with previous studies, we used fewer trials to produce higher reliability of ANSs. Meanwhile, we demonstrated a differentiated but integrated attention system. Specifically, the three networks are independent but mutually inhibited when two networks are operated simultaneously. The mutual inhibition was in line with the resource competition hypothesis. The high reliability of ANSs and directional INRs may shed light on the developmental, degenerative, and pathological mechanisms of attention networks and benefit the application of ANT in developmental and clinical studies.
Material and methods
Subjects
Fifty subjects took part in the experiment at extracurricular time (29/21 males/females, reported right-handed, ages ranged from 18–29 years with mean age of 20.34 ± 2.86). All participants had regular routines and were asked to have enough sleep and avoid from any drugs and alcohol in 12 hours before the experiment. All participants reported normal or correct-to-normal vision, without any medication, and neurological or psychiatric disorders. Six subjects were excluded from subsequent analyses due to following reasons: one for misunderstanding the introduction, two for procedure erroneous, and three for low accuracy (<80%) in any condition.
Ethics Statement
This study was approved by the research ethical committee of School of Life Science and Technology, University of Electronic Science and Technology of China. The methods were carried out complied with the tenets of the Declaration of Helsinki. All the participants gave their informed consents prior to their inclusion in the study.
Apparatus and Stimuli
The experiment was run on a Lenovo-PC computer with a 20-inch color screen monitor. E-Prime 2.0 software (http://www.pstnet.com; Psychology Software Tools, Inc) was used for programming, display of stimuli, and timing control. Responses were collected through the computer keyboard. All stimuli were black figures presented at the center of a screen on a gray background. Fixation was a plus sign and subtended 0.22° visual angle. The length of the whole target (five arrows and inter-arrow blank) was 3.1°. The length of each arrow was 0.53° visual angle. The spatial target was 2° from the fixation.
Procedure and design
All participants completed six blocks of the mixed design attention network test. The order of six blocks was randomized. Each effect block contained 4 practice trials and 48 experimental trials, while each interaction block included 4 practice trials and 96 experimental trials. Eighteen cue-target conditions were involved in the task. This design advanced our previous study12 to all directional INRs. Participants were seated 70 cm from the monitor and were asked to focus on the fixation throughout the experiment and, during practice. As shown in Fig. 4, each trial began with a fixation lasting for 400–1000 ms. A 100 ms fixation (for O, E and half of the trials of A) or asterisk (for half of the trials of A) was followed. After a 300 ms delay, the target (central or spatial, congruent or incongruent) was presented for 1700 ms or lasted until the participant pressed a key. Lastly, another delay was presented to insure the overall time of the trial from cue to the end of the trial was 3000 ms.
Figure 4.
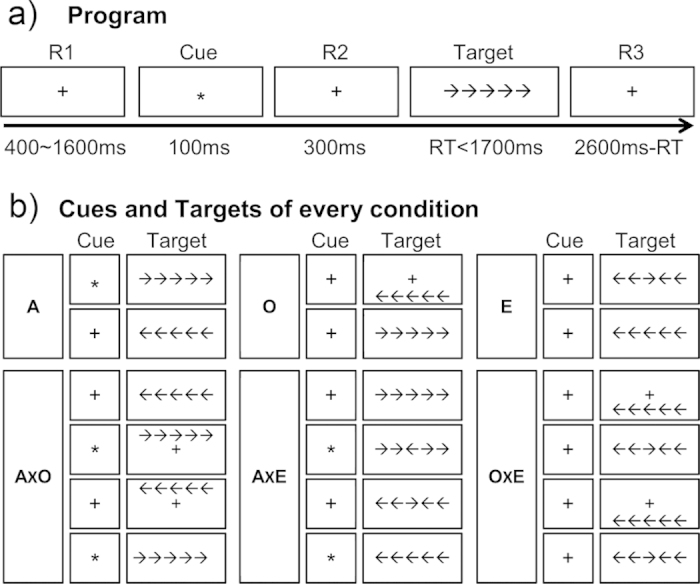
The program of the mixed design attention network test consisted of six blocks. The effects of alerting (A), orienting (O), and executive control (E) networks were tested in separated blocks to avoid interactions among them. The inter-network relationships were also assessed in independent blocks. For A and E, central targets were used to exclude the orienting effect; for O and E, cues were canceled to exclude the alerting effect; for A and O, incongruent targets were eliminated to avoid the conflict effect.
Instead of the conventional subtraction measure5,6, we used ratio scores to define the efficiency of attention networks and INRs. The ratio scores could avoid the baseline difference and isolate the attention system from the overall reaction time (RT)21,50. The ANSs are listed in equations (1, 2, 3) using conditions in effect blocks.
 |
 |
 |
The INRs are calculated using conditions in interaction conditions with equations (4, 5, 6, 7, 8, 9). This method allows us to test the direction of impact beyond interaction, promoting our understanding of the relationship among attention networks.
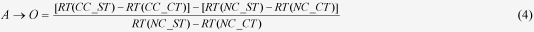 |
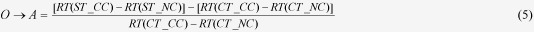 |
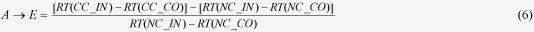 |
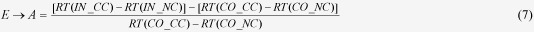 |
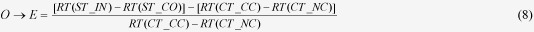 |
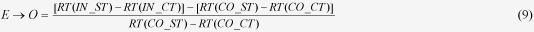 |
The abbreviations and the trials or effects they stand for are listed as follows: CC = central cue; NC = no cue; CT = central target; ST = spatial target; CO = congruent target; IN = incongruent target; A = alerting effect; O = orienting effect; E = executive control effect; X→Y =the influence from X to Y.
In these equations, the negative effect denoted beneficial in RT, whereas the positive effect represented cost in RT. This provided us an intuitive illumination of ANSs and INRs.
Statistical analysis
All ANSs and INRs were computed based on RTs. The efficiency of ANSs and INRs was tested with one-sample t-test. The independency of three networks was examined using repeated measures analysis of variance (ANOVA). The relationships among ANSs and between INRs of each pair of network were assessed by Pearson correlation. Furthermore, the split-half reliability of three ANSs was estimated with a Monte Carlo simulation. Specifically, for each ANS, the 24 trials of each condition in each subject were randomly split into two halves 10000 times. The ANS was calculated in each of the two halves. The reliability was computed based on the 10000 pairs of ANS in forty-four subjects. The final reliability was the mean of 10000 correlation coefficients.
Author Contributions
Y.F.W., X.J.J., J.H.Y. and H.F.C. designed the experiment; Y.F.W. and X.J.J. performed the experiment; Y.F.W., X.J.J., F.L., M.L.L. and Z.L.L. analyzed the data; Y.F.W., X.J.J., F.L., J.H.Y. and H.F.C. wrote the manuscript. All authors reviewed the manuscript.
Additional Information
How to cite this article: Wang, Y.-F. et al. Reliable Attention Network Scores and Mutually Inhibited Inter-network Relationships Revealed by Mixed Design and Non-orthogonal Method. Sci. Rep. 5, 10251; doi: 10.1038/srep10251 (2015).
Acknowledgments
The work is supported by the 973 project (No. 2012CB517901), the 863 project (No. SQ2015AA0201497), the Natural Science Foundation of China (Nos. 61125304, 81301279 and 81171406), the China Scholarship Council China (No. 2011607033), the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education China (No. A03003023901010), the Fundamental Research Funds for the Central Universities (No. ZYGX2013Z004), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120185110028).
References
- Raz A. & Buhle J. Typologies of attentional networks. Nat. Rev. Neurosci. 7, 367–379 (2006). [DOI] [PubMed] [Google Scholar]
- Posner M. I. & Petersen S. E. The attention system of the human brain. Annu. Rev. Neurosci. 13, 25–42 (1990). [DOI] [PubMed] [Google Scholar]
- Petersen S. E. & Posner M. I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 35, 73–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I. Imaging attention networks. NeuroImage 61, 450–456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. McCandliss B. D. Sommer T. Raz A. & Posner M. I. Testing the efficiency and independence of attentional networks. J. Cognitive Neurosci. 14, 340–347 (2002). [DOI] [PubMed] [Google Scholar]
- Fan J. et al. Testing the behavioral interaction and integration of attentional networks. Brain Cognition 70, 209–220 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas A. Lupianez J. Funes M. J. & Tudela P. Modulations among the alerting, orienting and executive control networks. Exp. Brain Res. 167, 27–37 (2005). [DOI] [PubMed] [Google Scholar]
- Greene D. J. et al. Measuring attention in the hemispheres: The lateralized attention network test (LANT). Brain Cognition 66, 21–31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I. Rothbart M. K. & Sheese B. E. Attention genes. Dev. Sci. 10, 24–29 (2007). [DOI] [PubMed] [Google Scholar]
- MacLeod J. W, et al. Appraising the ANT: Psychometric and theoretical considerations of the attention network test. Neuropsychol. 24, 637–651 (2010). [DOI] [PubMed] [Google Scholar]
- Habekost T. Petersen A. & Vangkilde S. Testing attention: Comparing the ANT with TVA-based assessment. Behav. Res. Methods 46, 81–94 (2014). [DOI] [PubMed] [Google Scholar]
- Wang Y-F. et al. A new method for computing attention network scores and relationships between attention networks. PLoS ONE 9, e89733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M. M. & Shore D. I. Mixing measures: testing an assumption of the attention network test. Atten., Percept, & Psycho. 73, 1096–1107 (2011). [DOI] [PubMed] [Google Scholar]
- Awh E. Belopolsky A. V. & Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn. Sci. 16, 437–443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E. & Dubis J. W. The mixed block/event-related design. NeuroImage 62, 1177–1184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. A.& Klein R. M. Isolating exogenous and endogenous modes of temporal attention. J. Exp. Psychol.: G. 142, 560–572 (2013). [DOI] [PubMed] [Google Scholar]
- Chica A. B. Bartolomeo P. & Lupiáñez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav. Brain Res. 237, 107–123 (2013). [DOI] [PubMed] [Google Scholar]
- Chica A. B. Sanabria D. Lupiáñez J. & Spence C. Comparing intramodal and crossmodal cuing in the endogenous orienting of spatial attention. Exp. Brain Res. 179, 353–364 (2007). [DOI] [PubMed] [Google Scholar]
- Macaluso E. Orienting of spatial attention and the interplay between the senses. Cortex 46, 282–297 (2010). [DOI] [PubMed] [Google Scholar]
- Roberts K. L. Summerfield A. Q. & Hall D. A. Presentation modality influences behavioral measures of alerting, orienting, and executive control. J. Int. Neuropsych Soc. 12, 485–492 (2006). [DOI] [PubMed] [Google Scholar]
- Westlye L. T. Grydeland H. Walhovd K. B. & Fjell A. M. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb. Cortex 21, 345–356 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Two-stage processing in automatic detection of emotional intensity: a scalp event-related potential study. Neuroreport. 24, 818–821 (2013). [DOI] [PubMed] [Google Scholar]
- Fjell A. M. et al. Multimodal imaging of the self-regulating developing brain. P. Natl. Acad Sci. USA 109, 19620–19625 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M. R. et al. Development of attentional networks in childhood. Neuropsychologia 42, 1029–1040 (2004). [DOI] [PubMed] [Google Scholar]
- Galvao-Carmona A. et al. Disentangling the attention network test: behavioral, event related potentials, and neural source analyses. Front hum. neurosci. 8, 1–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. I. et al. Attentional mechanisms of borderline personality disorder. P. Natl. Acad. Sci. USA 99, 16366–16370 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskin L. P. & White P. M. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology 21, 275–284 (2007). [DOI] [PubMed] [Google Scholar]
- van Donkelaar P. et al. Attentional deficits in concussion. Brain Injury 19, 1031–1039 (2005). [DOI] [PubMed] [Google Scholar]
- Nestor P. G. et al. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 90, 308–315 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. et al. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res. 78, 235–241 (2005). [DOI] [PubMed] [Google Scholar]
- Vázquez-Marrufo M. et al. Neural Correlates of Alerting and Orienting Impairment in Multiple Sclerosis Patients. PLoS ONE 9, e97226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva J. M.& Palva S. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. NeuroImage 62, 2201–2211 (2012). [DOI] [PubMed] [Google Scholar]
- Salthouse T. A.& Berish D. E. Correlates of within-person (across-occasion) variability in reaction time. Neuropsychology 19, 77–87 (2005). [DOI] [PubMed] [Google Scholar]
- Wang Y-F. et al. Steady-state BOLD response modulates low frequency neural oscillations. Sci. Rep.-UK 4, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RG. et al. Self-alert training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 46, 1379–1390 (2008). [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. The Attention Systems of the Human Brain in Attention Disorders After Right Brain Damage (ed. Bartolomeo P. ) 1–19Springer: London, 2014). [Google Scholar]
- Fuentes L. J. & Campoy G. The time course of alerting effect over orienting in the attention network test. Exp. Brain Res. 185, 667–672 (2008). [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D. Posner M. I. Relating the mechanisms of orienting and alerting. Neuropsychologia 35, 477–486 (1997). [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K. Carrasco M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat. Rev. Neurosci. 14, 188–200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta F. Lupiáñez J. & Chica A. B. When endogenous spatial attention improves conscious perception: Effects of alerting and bottom-up activation. Consciousness Cognition 23, 63–73 (2014). [DOI] [PubMed] [Google Scholar]
- Hayward D. A. & Ristic J. Measuring attention using the Posner cuing paradigm: the role of across and within trial target probabilities. Front Hum. Neurosci. 7, 1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böckler A. Alpay G.& Stürmer B. Accessory stimuli affect the emergence of conflict, not conflict control: A Simon-task ERP study. Exp. Psychol. 58, 102–109 (2011). [DOI] [PubMed] [Google Scholar]
- Fischer R. Plessow F. & Kiesel A. Auditory warning signals affect mechanisms of response selection: evidence from a Simon task. Exp. Psychol. 57, 89–97 (2010). [DOI] [PubMed] [Google Scholar]
- Fischer R. Plessow F.& Kiesel A. The effects of alerting signals in action control: activation of S–R associations or inhibition of executive control processes? Psychol. Res. 76, 317–328 (2012). [DOI] [PubMed] [Google Scholar]
- Weinbach N. & Henik A. Phasic alertness can modulate executive control by enhancing global processing of visual stimuli. Cognition 121, 454–458 (2011). [DOI] [PubMed] [Google Scholar]
- Weinbach N.& Henik A. The relationship between alertness and executive control. J. Exp. Psychol.: Human. 38, 1530–1540 (2012). [DOI] [PubMed] [Google Scholar]
- Cools R. & D’Esposito M. Inverted-U–Shaped Dopamine actions on human working memory and cognitive control. Biol. Psychiat. 69, e113–e125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias E. et al. The influence of alertness on spatial and nonspatial components of visual attention. J. Exp. Psychol.: Human 36, 38–56 (2010). [DOI] [PubMed] [Google Scholar]
- Ishigami Y. Fisk J. D. Wojtowicz M. Klein R. M. Repeated measurement of the attention components of patients with multiple sclerosis using the Attention Network Test-Interaction (ANT-I): Stability, isolability, robustness, and reliability. J. Neurosci. Methods 216, 1–9 (2013). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. Spontaneous neuronal activity predicts intersubject variations in executive control of attention. Neuroscience 263, 181–192 (2014). [DOI] [PubMed] [Google Scholar]


