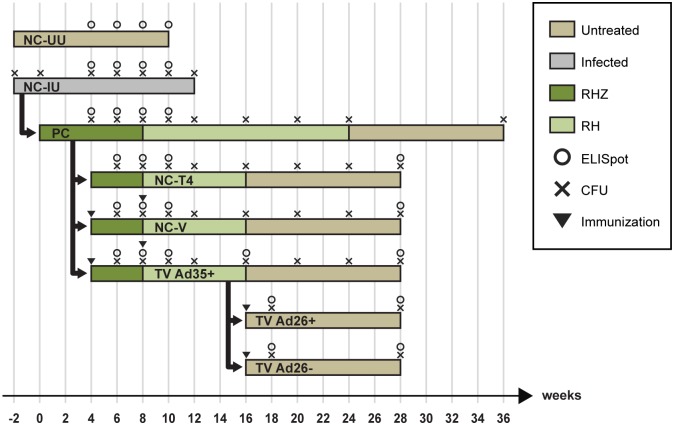
Fig 1. Schematic of study design.
420 mice were divided into animals that were uninfected (NC-UU) (20 mice) and those that were infected with M.tb at week -2 (NC-IU) (400 mice). At week 0, 365 mice from NC-IU were removed to create the PC cohort that was initiated on therapy with R, H and Z via oral gavage, 7 days per week. The remaining NC-IU animals were left untreated up to week 12, when they became moribund and were sacrificed. At week 4, 284 animals from the PC cohort were randomized into groups NC-T4, NC-V and TV Ad35+. At this time point, mice in the NC-V and TV Ad35+ groups received intramuscular immunotherapy with 1010 viral particles of Ad35 empty vector or Ad35-TBS vaccine, which were respectively boosted at week 8. Animals in the PC, NC-T4, NC-V and TV Ad35+ groups were switched to therapy with R and H at week 8 which lasted to week 16, except for the PC group which received therapy up to week 24. At week 16, when therapy was shortened, 56 mice from the TV Ad35+ group were removed and divided into 2 further groups, TV Ad26+ and TV Ad26- which received immunotherapy with Ad26-TBS and Ad26, respectively. All animals were followed through for 3 months following therapy cessation at week 16 and week 24 (PC). Animals were subsequently sacrificed for the final lung CFU enumeration. Multiplex analysis on lung samples were carried out at times indicated for CFU counting, from week 4 onwards.