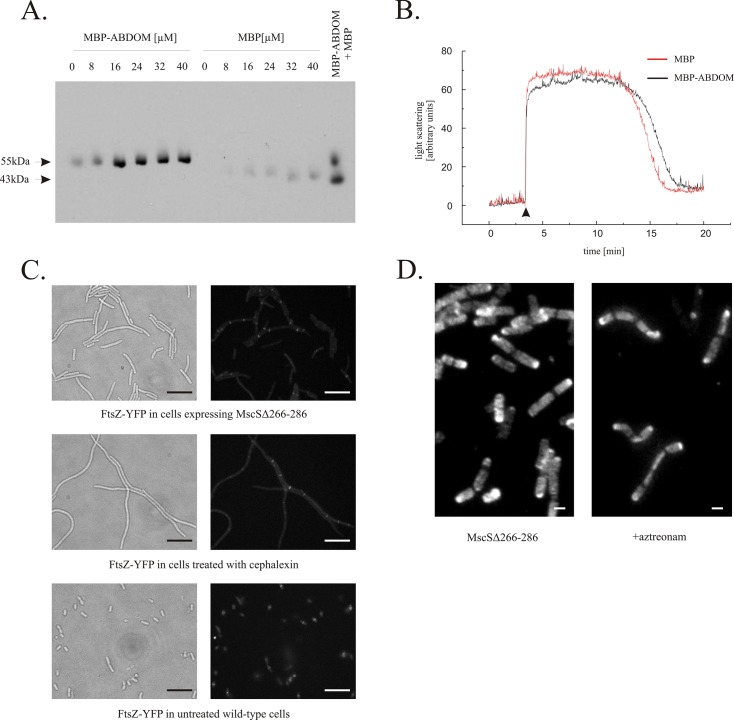
Fig 2. ABDOM binds FtsZ but does not interfere with its polymerization neither in vitro nor in vivo.
A. Purified MBP-ABDOM bound assembled FtsZ in vitro. The MBP-ABDOM fusion protein, but not MBP alone, co-sedimented with polymerized FtsZ. Fixed concentration of FtsZ (16 μM) was co-sedimented by addition of 1mM GTP in the presence of increasing concentration of MBP or MBP-ABDOM. For reference, equal amounts of MBP-ABDOM and MBP supernatant without FtsZ are shown in rightmost lane. Proteins were detected with anti-MBP antibody. B. MBP-ABDOM had no effect on FtsZ polymerization/depolymerization in vitro, as detected by 90° angle light scattering. FtsZ was polymerized after addition of 1 mM GTP (arrow) in the presence of either MBP-ABDOM (black line) or MBP (red line) C. Filaments produced by MscSΔ266–286 expression contain multiple nonfunctional Z-rings (upper row), which resemble Z-rings arrested by the cephalexin inhibition of PBP3 (middle row), control cells (lower row). FtsZ-YFP was expressed to visualize Z-rings. Scale bar represents 10 μm. D. Peptidoglycan synthesis detected by immunofluorescent D-cysteine labeling. Overexpression of MscSΔ266–286 resulted in cell filamentation, with clear dark rings of newly synthesized murein in septal areas (left). A similar pattern of murein segregation was observed in cells treated with 1μg/ml aztreonam, a selective inhibitor of PBP3 whose septal localization depends on functional Z-rings (right). Experiments were carried out over one cell cycle in D-cysteine, followed by 30 min. chase. The labeled, older murein is seen as bright spots, while newly made murein is seen as dark areas. Scale bar represents 1 μm.