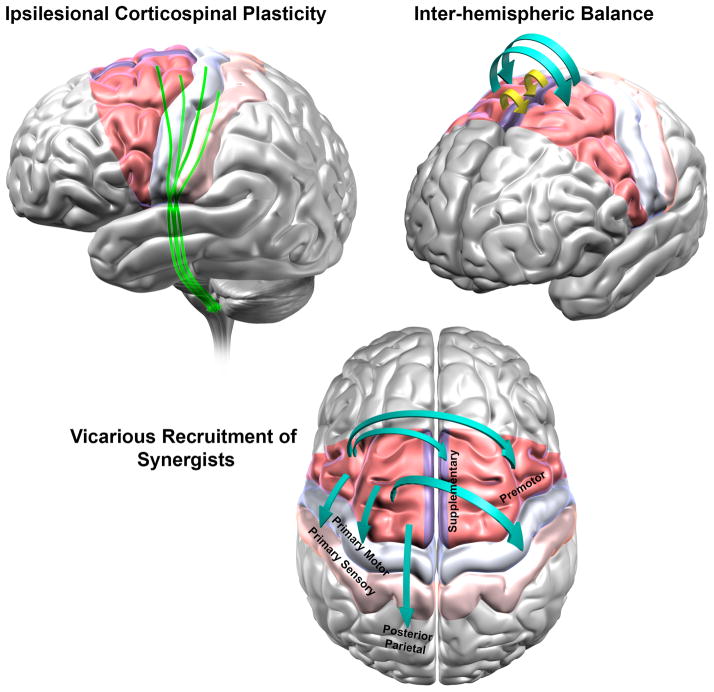
Fig. 3. Why would stimulation of ipsilesional PMA be able to affect most mechanisms of clinical recovery in stroke?
The figure shows the anatomical region of primary motor cortex (M1) and the expanse of PMA [red = Lateral Premotor Areas, purple = Medial Premotor as Supplementary Motor Area, and Cingulate Motor Area, which cannot be shown in this view). PMA constitute more than 60% of the frontal cortex that project to the spinal cord. Medial wall, such as Supplementary Motor Area and medial premotor cortex, receive arterial supply from a source that is different from the commonly infarcted middle cerebral artery that supplies M1. Thus, PMA would have greater probability of survival than M1 and their constituent regions could serve as effective substitutes for M1.
Based on evidence from three of our studies (Carey and others 2007; Cunningham and others 2013b; Plow [nee (Bhatt)] and others 2007) and neuroanatomic, physiologic, and functional evidence from that of several others, we present a hypothetical model of how and why stimulation of ipsilesional PMA would be most effective in modulating majority of mechanisms of human stroke recovery in rehabilitation. Clinical recovery of paretic hand function is believed to occur via at least 3 adaptive processes- corticospinal plasticity (A) return of balance between excitability of ipsilesional and contralesional motor regions (B) and vicarious recruitment of widespread frontal and parietal synergistic regions (C). Stimulating PMA would be able to facilitate (A) because they contribute CST, as extensively as M1, forming direct, parallel output to spinal cord, independent of M1. In stroke, their CST can exhibit plasticity via axonal sprouting and/or cortico-cortical facilitation of CST from M1. Stimulation of PMA would aid (B) because they possess abundant callossal connections, both homotopic and heterotopic, which are far more extensive than between M1 and its homologue. In fact, in stroke, their callossal connectivity enhances i.e. undergoes adaptive plasticity, with restorative therapy. Their stimulation would finally also facilitate (C) because they have extensive functional connectivity with ipsilateral posterior parietal that enhances in recovery, structural connectivity with ipsilateral sensory cortex that rewires following stroke affecting M1, and strong structural connectivity with ipsilateral and contralateral motor areas (M1 and other PMA). With their stimulation, they would be able to help recruit widespread synergists in motor function.