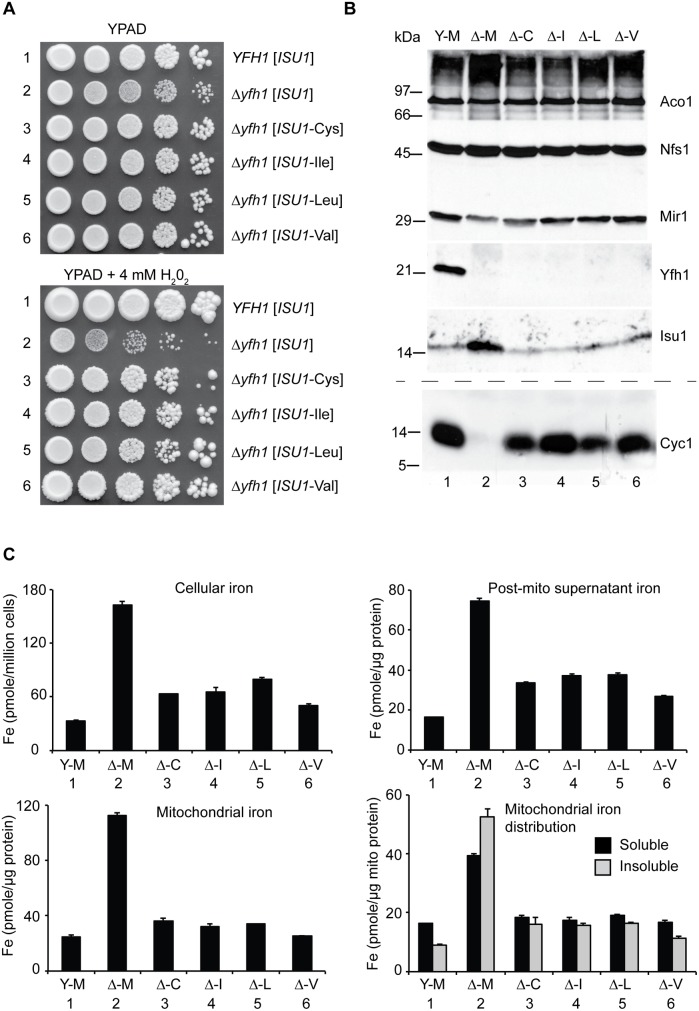
Fig 2. Characterization and comparison of frataxin-bypass substitutions of ISU1 amino acid 141: ISU1-Cys, ISU1-Ile, ISU1-Leu and ISU1-Val.
(A) Growth defects in Δyfh1 are corrected by the frataxin-bypass suppressors. The strains YFH1 [ISU1] (Y-M), Δyfh1 [ISU1] (Δ-M), and suppressor strains Δyfh1 [ISU1-Cys] (Δ-C), Δyfh1 [ISU1-Ile] (Δ-I), Δyfh1 [ISU1-Leu] (Δ-L), and Δyfh1 [ISU1-Val] (Δ-V), were grown in an argon-filled chamber, and serial dilutions were spotted on YPAD agar, without or with 4 mM hydrogen peroxide. (B) Mitochondrial protein levels by immunoblotting. Strains were inoculated from the argon-filled chamber into aerobic defined raffinose medium for 16–24 h prior to isolating mitochondria. Mitochondrial proteins (100 μg) were separated by SDS-PAGE and transferred to nitrocellulose. Strips were cut from a single blot and probed with the indicated antibodies against mitochondrial proteins (Aco1, Nfs1, Mir1, Yfh1, and Isu1). Cytochrome c (Cyc1) was analyzed using a duplicate gel. (C) Iron homeostasis in the frataxin-bypass suppressors. The indicated strains were grown in air for six doublings in defined raffinose medium in the presence of 10 μM 55Fe ascorbate. Cells were washed free of radioactive iron and separated into mitochondria and post-mitochondrial supernatant. An aliquot of mitochondria was lysed in the presence of 0.1% Triton X-100 for 10 min at room temperature, and supernatant (soluble) and pellet (insoluble) fractions were separated by centrifugation at 20,000 x g for 30 min at 4°C. Iron content was reported for whole cells (pmol per million cells), or various cellular fractions (pmol per micrograms protein).