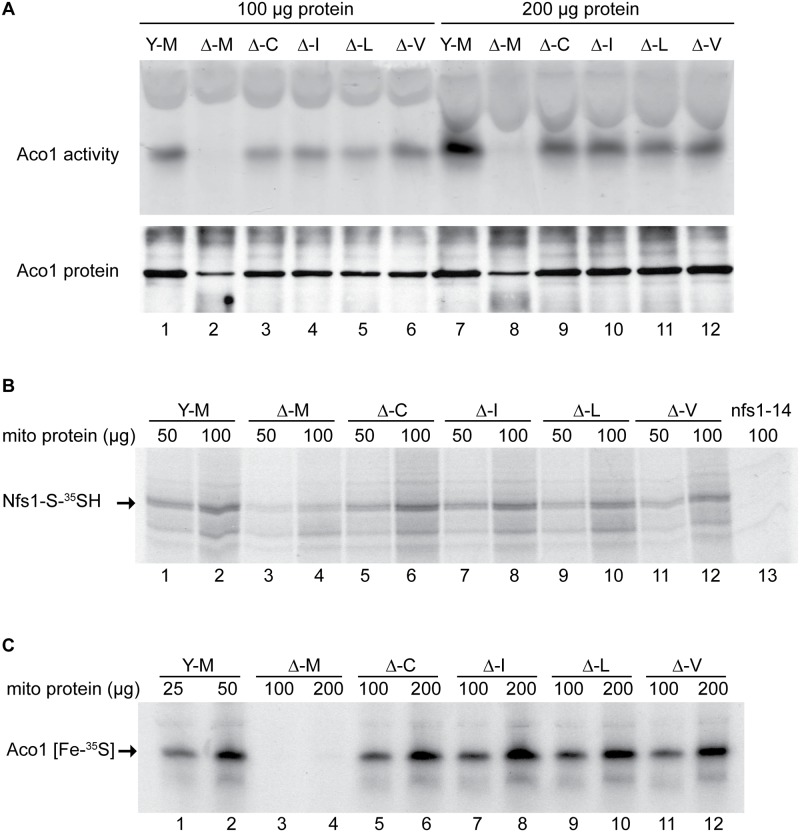
Fig 3. Assessment of Fe-S cluster assembly in mitochondria isolated from frataxin-bypass mutants.
The same strains as in Fig 2 were assayed as follows: (A) Aconitase activity by in-gel assay. Mitochondria were lysed and 100 μg (lanes 1–6) or 200 μg (lanes 7–12) proteins were separated by native gel and developed using reagents that reflect aconitase activity (upper panel). The same lysates were separated by SDS-PAGE and analyzed by immunoblotting with anti-aconitase antibody to show the level of total aconitase protein (lower panel). (B) Persulfide formation on Nfs1 in mitochondria. Isolated and intact mitochondria were depleted of nucleotides and NADH by incubation at 30°C for 10 min. Either 50 μg or 100 μg protein equivalents were labeled for 15 min with 35S-cysteine. Samples were diluted with buffer, mitochondria were recovered by centrifugation, and total proteins were analyzed by non-reducing SDS-PAGE and autoradiography. The persulfide Nfs1-S-35SH is indicated by an arrow. Mitochondria from the nfs1-14 mutant was included as a negative control (lane 13), because this hypomorphic Nfs1 mutant has negligible persulfide-forming activity [16]. (C) New Fe-S cluster synthesis on apoaconitase in isolated mitochondria. Isolated and intact mitochondria, were labeled with 35S-cysteine in the presence of added 4 mM ATP, 1 mM GTP, 5 mM NADH and 10 μM ferrous ascorbate for 30 min at 30°C. Mitochondria were recovered and membranes were ruptured. After centrifugation, the supernatant fractions containing soluble mitochondrial proteins (corresponding to 25 or 50 μg for YFH1 [ISU1] and 100 or 200 μg for the other strains) were separated by native gel prior to autoradiography. The arrow indicates the aconitase protein containing newly made radiolabeled [Fe-35S] clusters.