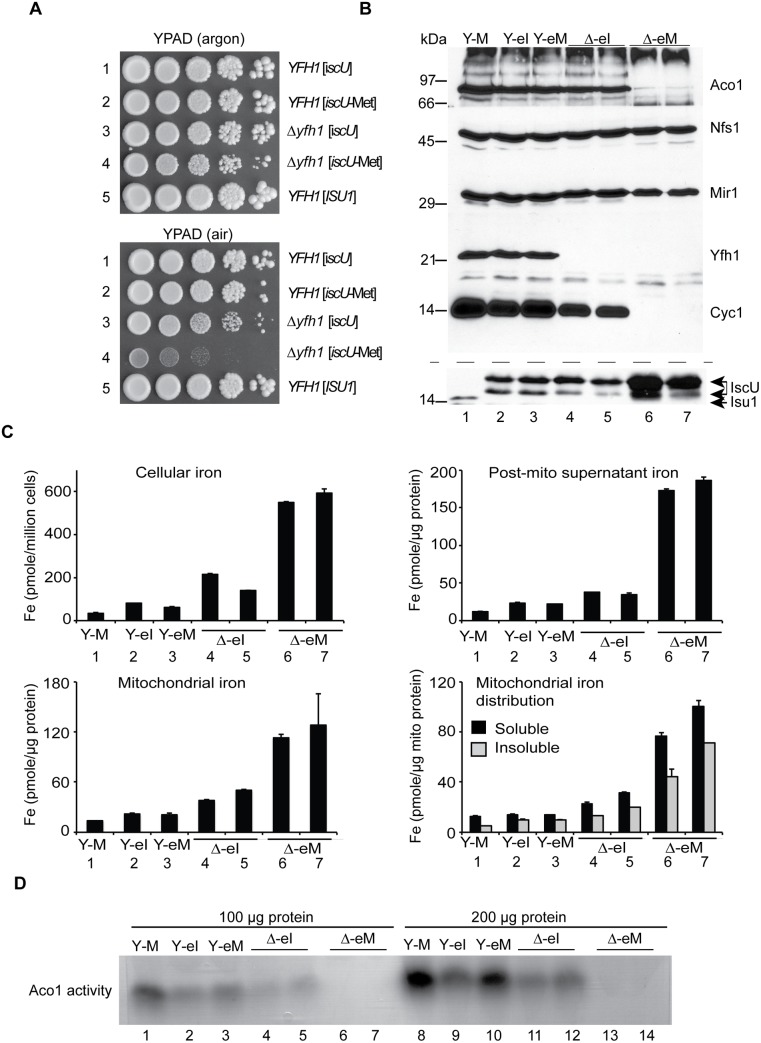
Fig 5. E. coli iscU or iscU-Met targeted to yeast mitochondria.
(A) Growth phenotypes in argon or in air. Strains with mitochondrial targeted E. coli IscU proteins indicated in the figure were grown on YPAD in argon or in air for 3 days and photographed. Strain YFH1 [ISU1] was included as a control. (B) Mitochondrial protein levels by immunoblotting. The following strains were evaluated: 1) YFH1 [ISU1] or Y-M, 2) YFH1 [iscU] or Y-eI, 3) YFH1 [iscU-Met] or Y-eM, 4) Δyfh1 [iscU] or Δ-eI clone 1, 5) Δyfh1 [iscU] or Δ-eI clone 2, 6) Δyfh1 [iscU-Met] clone 1 or Δ-eM, and 7) Δyfh1 [iscU-Met] clone 2 or Δ-eM. Cultures were inoculated into defined raffinose medium bubbled with argon. After an initial growth period the cultures were shifted to air. The doubling time of the Δyfh1 [iscU-Met] clones in air started at 2.5 h but prolonged to greater than 8 h, necessitating a longer growth period prior to harvesting these cells. Mitochondrial proteins were separated by SDS-PAGE, and transferred to nitrocellulose, which was cut horizontally into segments and probed with various antibodies against various mitochondrial proteins (Aco1, Nfs1, Mir1, Yfh1, and Cyc1). The segment used for anti-Cyc1 was stripped and reprobed with anti-Isu1. The antibody to the yeast Isu1 was used to detect the E. coli IscU. The two bands attributed to E. coli IscU are indicated by a double arrow, and yeast Isu1 is indicated by a single arrow. (C) Iron homeostasis. The indicated strains were precultured in argon-bubbled raffinose medium and then shifted to air in the presence of 10 μM 55Fe ascorbate. Following growth to a density of 2 x 107 cells/ml, cells were harvested. Total cellular iron and iron in cellular fractions (post-mitochondrial supernatant, mitochondria, and soluble or insoluble fractions of mitochondria) were measured as in Fig 2C. (D) Aconitase activity. Mitochondria from the indicated strains were lysed, and 100 μg (lanes 1–7) or 200 μg (lanes 8–14) of proteins were separated by native gel and analyzed for aconitase activity using the in-gel assay.