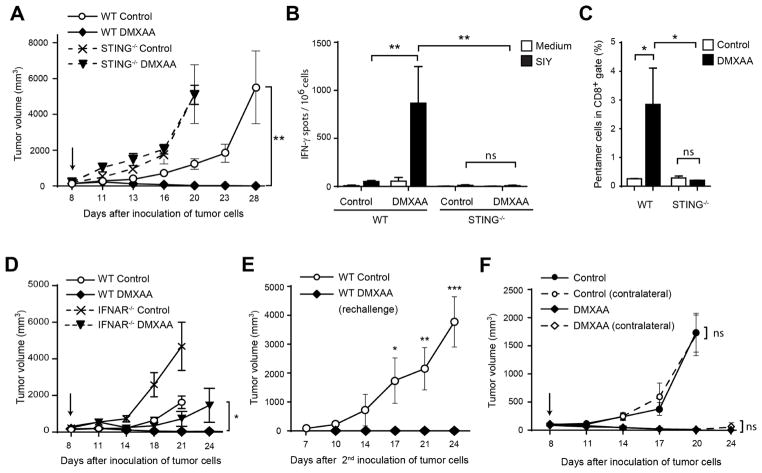
Figure 2. Rejection of tumors in response to DMXAA is STING-dependent.
(A) WT or STING−/− C57BL/6 mice were inoculated with 106 B16.SIY cells in the left flank. When tumor volumes were 100–200 mm3 they received a single IT dose of 500 μg of DMXAA or saline. Tumor volume was measured at the indicated time points (n=5). (B–C) WT or STING−/− C57BL/6 mice (n=5) were treated as in (A) and 5 days later splenocytes were harvested and re-stimulated in vitro in the presence of culture medium or soluble SIY peptide for 16 hours. The frequency of tumor-specific IFN-γ–producing cells was assessed by ELISPOT (B), and the percentage of SIY-specific CD8+ T cells was assessed by staining splenocytes with antibodies against TCRβ, CD4, CD8 and SIY pentamer (C). Cells were acquired in the LSRII-Blue cytometer and analyzed with FlowJo software. Results are shown as mean ± s.e.m. * P < 0.05; ** P < 0.01. (D) WT or IFNAR−/− C57BL/6 mice were inoculated with 106 B16.SIY cells in the left flank (n=5). When tumor volumes were 100–200 mm3 they received a single IT dose of 500 μg of DMXAA or saline. Tumor volume was measured at the indicated time points. (E) WT mice that had rejected B16.SIY tumors were re-challenged with 106 B16.SIY in the contralateral flank. Naïve mice were used as controls. Tumor size was measured at the indicated time points. (F) WT mice were inoculated with 106 B16.SIY cells in the left and the right flanks (n=5). When tumor volumes were 100–200 mm3, 500 μg of DMXAA or saline was injected IT in the right flank only, and tumor volume was measured at the indicated time points. Data are representative of at least three independent experiments, or two independent experiments for the contralateral tumor model. Results are shown as mean tumor volume ± s.e.m. * P < 0.5; ** P < 0.01; *** P < 0.001. ns, not significant.