Fig 4.
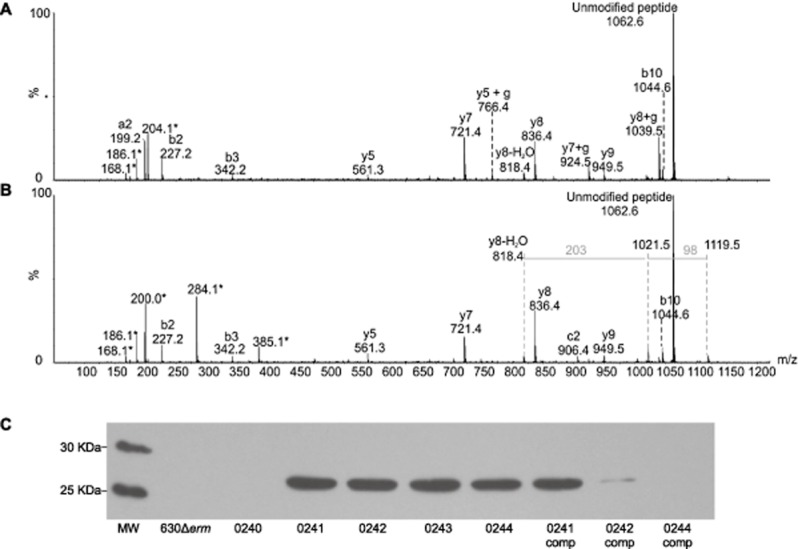
Mass spectrometry analyses of C. difficile flagellin tryptic glycopeptides from strain 630Δerm CD0243 mutant. Peptide MS/MS spectra were obtained from in gel digestion of protein bands from glycine extraction of whole cells.A. MS/MS spectrum of C. difficile flagellin glycopeptide LLDGTSSTIR. The unmodified peptide ion was observed at m/z 1062, with low intensity peptide type y and b ions confirming the peptide sequence. A moderately intense HexNAc oxonium ion was observed at m/z 204, with sequential dehydration giving rise to glycan related ions at m/z 186 and 168. Glycan related fragment ions are indicated with an asterisk.B. MS/MS spectrum of C. difficile flagellin glycopeptide LLDGTSSTIR, modified with a glycan of 384 Da. The MS/MS spectrum shows the typical peptide fragment ions and an additional, with a glycan oxonium ion at m/z 385. Glycan related ions were also observed at m/z 284, 186 and 168. In addition, in the higher m/z region of the spectrum neutral losses of 98 and 203 Da were observed. Together these data suggest the 384 Da glycan moiety is similar to the 398 Da wild type sugar, with the absence of a methyl group. C, Flagellin from 630Δerm and the modification mutants were analysed by Western blot, probing with an anti-β-O-GlcNAc antibody (MW = molecular weight, gene numbers below indicate mutation, comp = in trans complementation of mutant). With the exception of the CD0240 mutant, the flagellin of all mutants was found react with the anti-β-O-GlcNAc antibody, however the parental strain 630Δerm and the CD0244 complement did not.
