Abstract
Many women with ductal carcinoma in situ (DCIS) are treated with extensive surgery, radiation, and hormone therapy due to the inability to monitor the disease and to determine which cases will progress to invasive cancer. We assessed the safety and feasibility of administering chemotherapy directly into DCIS-containing ducts in 13 women before definitive surgery. The treatment was safe, feasible, and well tolerated, supporting further development of this strategy for management of DCIS.
Introduction
Ductal carcinoma in situ (DCIS) is a noninvasive breast cancer wherein malignant cells are confined within a ductal lobular unit. Although less than half the cases of DCIS will progress to invasive disease, most women are treated aggressively with surgery, radiation, and/or hormone therapy due to the inability to clinically evaluate the extent and location of the disease. Intraductal therapy, in which a drug is administered directly into the mammary duct through the nipple, is a promising approach for treating DCIS, but the feasibility of instilling drug into a diseased duct has not been established.
Patients and Methods
Four to 6 weeks before their scheduled surgery, 13 women diagnosed with DCIS were subjected to cannulation of the affected duct. After both the absence of perforation and presence of dye in the duct were confirmed by ductogram, pegylated liposomal doxorubicin was instilled. Histopathologic assessment was performed after surgery to assess the treatment effects.
Results
Of the 13 women enrolled in the study, 6 had their DCIS duct successfully cannulated without perforation and instilled with the drug. The treatment was well tolerated, and no serious adverse events have been reported. Biomarker studies indicated a general decrease in Ki-67 levels but an increase in annexin-1 and 8-hydroxydeoxyguanosine in the lumen of DCIS-containing ducts, which suggests a local response to pegylated liposomal doxorubicin treatment.
Conclusions
Intraductal therapy offers a nonsurgical strategy to treat DCIS at the site of disease, potentially minimizing the adverse effects of systemic treatment while preventing development of invasive cancer.
Keywords: Doxil, Ductal carcinoma in situ, Local therapy, Mammary duct, Pegylated liposomal doxorubicin
Introduction
The majority of breast cancer begins in the epithelial lining of the milk ducts.1,2 Ductal carcinoma in situ (DCIS) is a noninvasive breast cancer in which malignant breast ductal epithelial cells have clonally proliferated and accumulated within the lumen of a mammary duct without invasion through the basement membrane.3–6 DCIS is considered an early step in the progression to invasive cancer, and the rationale for treating DCIS is to prevent this progression. The advent of mammographic screening has led to a dramatic increase in the incidence of DCIS,7 and results of a number studies suggest that between 14% and 53% of cases may progress to invasive cancer over a period of 10 or more years.8–12 Unfortunately, there is at present no way of knowing which in situ cancers will progress to invasive cancer, which results in the view that all DCIS need to be aggressively treated. Treatment approaches, which typically include some variation of lumpectomy or mastectomy combined with radiation and systemic treatment with hormonal drugs, also exhibit a wide range of damaging physical and emotional effects, all in an effort to treat a local problem that may not have ever caused any harm.
The nature of DCIS makes it difficult to treat. Because DCIS originates in the lining of the breast ducts, it can potentially spread widely through the arborizing path of the duct, unmarked by the particular patterns of microcalcifications that herald its presence on mammograms.13 This has been demonstrated in studies by using techniques of whole breast sectioning such as Holland et al7 and, more recently, Mai et al,14 who concluded that the in situ component is most often located in a single ductal tree or lobe. Tot,13 in his theory of the “sick lobe,” postulated that DCIS is a disease in which simultaneously or asynchronously appearing, often multiple, in situ tumor foci are localized within a single duct or lobe as opposed to being distributed among multiple ducts throughout the breast. Our inability to image the extent of disease before surgery or to identify it during surgery hinders the surgeon’s ability to do a precise resection. To this point, local recurrences usually emerge in areas missed by the initial excision rather than new disease.15 This leads to repeated operations to clear margins, poor cosmetic outcomes, and even mastectomy.
Intraductal therapy, in which drugs are instilled directly into a diseased duct through its nipple orifice, offers an alternate treatment strategy for DCIS.16,17 Similar local treatment approaches have found success in other types of cancer, such as intravesical treatment of bladder cancer18 and intraperitoneal treatment of ovarian cancer.19 To this end, intraductal therapy for breast cancer prevention and treatment has proved promising in animal models. For example, results of recent studies have shown that rats with N-methyl N′-nitrosourea-induced breast tumors exhibited significant reduction in tumor formation upon intraductal treatment with various anticancer agents, including paclitaxel, pegylated liposomal doxorubicin (PLD), 4-hydroxytamoxifen, carboplatin, methotrexate, nanoparticle albumin-bound paclitaxel and 5-fluorouracil, with minimal toxic effects.20–22 Similarly, human epidermal growth factor receptor 2/neu transgenic mice, which spontaneously develop neu overex-pressing multifocal mammary adenocarcinoma beginning at approximately 4–5 months of age, exhibited higher levels of tumor regression and prevention of tumor development upon intraductal PLD treatment than mice that received intravenous PLD.21
In translating intraductal therapy from animal models to humans, it is important to consider the differing anatomy between the rodent and human mammary gland. Although rats have 12 teats with 1 duct per teat, the human breast contains 5 to 9 ducts that all exit separately within a single nipple.23 In women, standard radiologic ductography has demonstrated the ability to cannulate the nipple and instill fluid into a ductal system, but it has not been clear that the dye will access the terminal duct lobular units where cancer is thought to begin. Goulet et al24 showed this elegantly when they instilled encapsulated epirubicin, a naturally fluorescent anthracycline, into a breast duct in previously removed breasts and showed the presence of intact epirubicin in the terminal lobules of 8 of 13 specimens. In addition, by analyzing horizontal sections under the nipple and throughout the breast, Love and Barsky23 demonstrated that water-insoluble dye instilled into 13 detached breasts reached the ductal lobular units.
Fueled by these promising preclinical and ex vivo intraductal studies, 2 clinical studies have been conducted recently to establish the safety and feasibility of intraductal administration of chemotherapy in women awaiting mastectomy. In 2011, Stearns et al22 conducted a phase I dose escalation study in which PLD was administered into a single duct in 15 premastectomy women diagnosed with invasive cancer. Pharmacokinetics showed that the plasma concentrations as early as 4 hours and up to 2 weeks after drug administration were lower in women who received intraductal PLD compared with those who received the agent intravenously. Conversely, drug concentrations in the breast were considerably higher in women who received intraductal PLD compared with those administered intravenous PLD. In a separate study, Love et al25 instilled 1 of 2 drugs, carboplatin or PLD, into 5 to 8 ducts per breast in 30 women 2 to 3 days before mastectomy. There were no adverse events, and both drugs were found to cause dose-dependent effects on ductal histology. Both of these studies demonstrated the safety and feasibility of intraductal therapy, which warrant further exploration of this approach.
Our hypothesis is that it should be possible to eliminate precancerous disease through directed intraductal therapy with minimal local and systemic adverse effects. Although DCIS would be the perfect setting for intraductal therapy, questions remain as to the ability to identify the correct duct as well as whether the drug would be able to be delivered into a duct with disease. In this study, we strove to demonstrate the feasibility of cannulating a specific DCIS-containing duct and instilling a cytotoxic agent before surgery. Because there is no accurate way to delineate the extent of DCIS before surgery, and, therefore, to prove a reduction in the extent of disease, we explored several potential markers that might be able to demonstrate an effect of our treatment, including histology, immunohisto-chemical markers, and breast magnetic resonance imaging (MRI). By demonstrating the feasibility of this novel local delivery approach in women with DCIS before surgery, we hope to lay the groundwork for establishing a more precise and less morbid approach to a common clinical problem, the local treatment of DCIS.
Patients and Methods
Subjects
This study was performed in accordance with the Declaration of Helsinki and was a Western Institutional Review Board approved study. Informed consent was obtained from all 13 subjects, all women, over the age of 18 years, diagnosed with pure DCIS on a core needle biopsy within 30 days of the procedure, had no prior treatment (surgery or radiation) to the recently diagnosed breast, had mammographic microcalcifications limited to 1 ductal system or 1 quadrant of breast, and were able to undergo necessary surgery. Subjects with any pathologic invasive or microinvasive disease in the affected breast, had received chemotherapy in the past 12 months, were pregnant, had any subareolar breast surgery to the affected breast, were a subject in a research protocol to evaluate an unapproved new drug, or were unwilling to sign informed consent were excluded from the study.
The original plan had been to study 30 women with this approach; however, midway through the study, we received new preclinical data that questioned the long-term safety of using PLD intraductally.26 although all the women in our study underwent surgical removal of the treated tissue, the data safety monitoring board decided that the study should be terminated prematurely.
Administration of PLD into the DCIS-containing Duct
Six to 8 weeks before scheduled surgery, baseline evaluations were conducted and included a standard history, physical examination, complete blood cell count, a pregnancy test if appropriate, and a unilateral breast MRI. Local anesthesia (lidocaine or Marcaine, Hospira, Lake Forest, IL) was administered into the nipple.27 Our previous work showed that the ductal orifices are arranged in an inner and outer circle and that the most likely location of the orifice of the affected duct is in fact easily predicted.23 To confirm that the correct duct had been cannulated and that there was no perforation, a small amount of premeasured contrast material was instilled into the ductal orifice, which enabled a ductogram. If the duct that was cannulated was not the duct with DCIS, then 2 additional attempts were made to cannulate the correct duct. If this still was unsuccessful, then the case was canceled. If any extravasation of the dye was seen, then the procedure was aborted and the woman did not continue with the study. Once cannulation of the correct duct and the absence of perforation were confirmed, 10 mL of saline solution or of 20 mg/mL PLD was instilled into the duct. Although, in the initial study design, 3 women were to be randomized to serve as controls and receive saline solution only, because the study was terminated after 13 subjects, only 1 woman served as a saline solution control. The subjects were blinded to whether they were receiving the drug or the saline solution. The patients were monitored for local and systemic adverse effects during and after instillation. After the procedure, the catheter was removed, the duct location was noted, and the nipple was covered by an occlusive dressing for the first 24 hours. The patients were monitored at 1 hour, 4 hours, 8 hours, and then 24 hours followed by weekly monitoring until the time of surgery.
Surgery was scheduled for 4 to 6 weeks after the procedure. Two to 3 days before the planned surgery, the women underwent a unilateral mammogram and breast MRI of the affected breast. The patients were followed every 6 months with clinical examination and breast imaging of the treated breast, and were followed-up by their breast surgeon for a minimum of 2 years, as is standard in all cases of DCIS.
Histopathologic and Immunohistochemical Analysis
Conventional histopathologic examination of the tissue sample was performed on hematoxylin and eosin–stained slides as usual, with specific attention paid to observe inflammatory responses and necrosis in tumor and nontumor areas as previously described.25 For each case, immunohistochemical analysis was performed on 2 representative paraffin-embedded tissue blocks, one from a region that contained DCIS and one from a noncancerous area. For the staining, 4-μm-thick tissue sections were prepared. The sections were first heated to 56°C for 20 minutes, followed by deparaffinization in xylene. The sections were then rehydrated in graded alcohols, and endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol at room temperature. The sections were then placed in a 95°C solution of 0.01 M sodium citrate buffer (pH 7.0) for antigen retrieval. Protein blocking was accomplished through application of 5% normal horse serum for 30 minutes. Endogenous biotin was then blocked with sequential application of avidin D and then biotin. The sections were then incubated for 1 hour with various primary antibodies at room temperature. Primary anti-8-hydroxydeoxyguanos-ine (8-OHdG) was a monoclonal antibody purchased from JaICA, Japan, anti-Ki-67 monoclonal antibody was purchased from DAKO, Carpenteria, CA, and anti-annexin 1 (ANX1) was a monoclonal antibody immunoglobulin G1 purchased from Abcam, Cambridge, MA. For anti-8-OHdG and anti-Ki-67, a 1:50 dilution was used, and, for anti-ANX1, a 1:750 dilution was used. After washing, bio-tinylated horse antimouse immunoglobulin G was applied for 30 minutes at room temperature. Next, the avidin-biotin complex was applied for 25 minutes and 3,3′-diaminobenzidine (DAKO) was used as the chromogen. Tris-buffered saline solution and Tween 20 buffer at pH 7.4 was used for all intermediate wash steps, and a moist humidity chamber was used for prolonged incubations. The sections were counterstained with Harris hematoxylin, followed by dehydration and mounting. A negative control section was prepared exactly in the same manner except omitting the primary antibody. Immunohistochemical stained slides were examined independently and separately by trained pathologists (JYR). The staining intensities (graded from 0 to 3) and percentage of staining for each staining grade were recorded separately in DCIS and non-DCIS areas.
MRI
Patients with DCIS underwent dynamic contrast-enhanced MRI of the breast before and after intraductal therapy by using standard clinical protocols. Image evaluation included assessment of image quality and quantitative measurement of DCIS extent. Image quality was rated as excellent, good, or poor. Image artifacts (ringing, dark borders, inhomogeneous fat-suppression) were noted where present and were ranked as mild, moderate, or marked. Quantitative measurements included DCIS longest diameter, DCIS volume, and contrast kinetics. The longest diameter of DCIS lesions was measured on subtracted images (early postcontrast minus precontrast dynamic contrast-enhanced images). Initial contrast uptake was rated as slow, medium, or fast. The presence of contrast washout was assessed as yes or no. Computer analysis by using customized software was used to measure tumor volume in cubic centimeters.
Results
Administration of PLD into the DCIS-Containing Duct
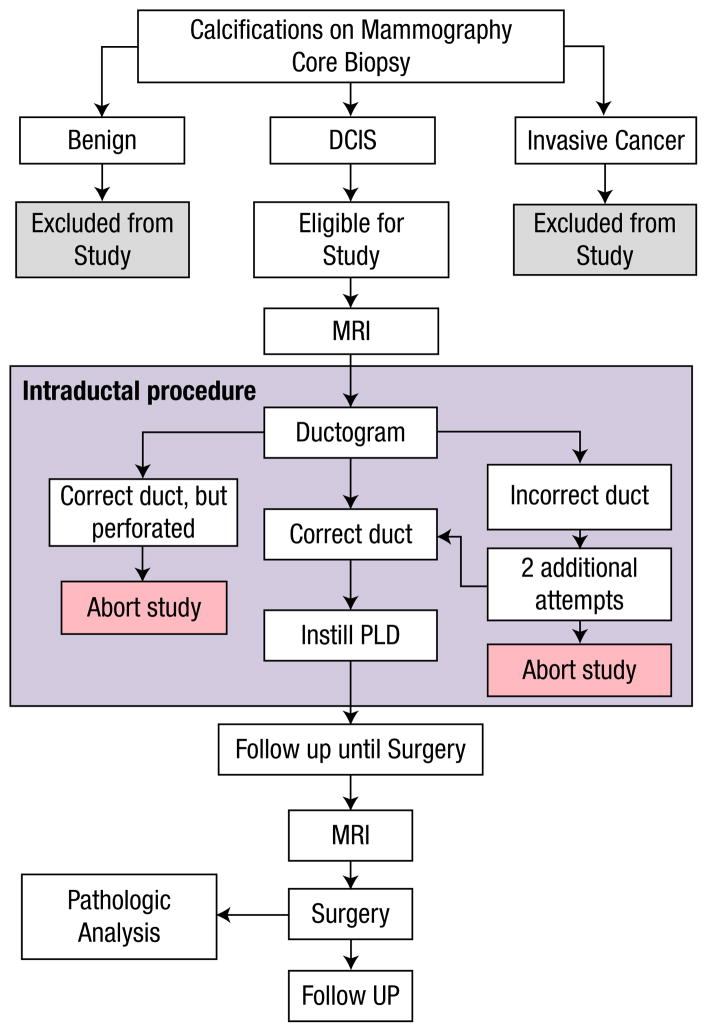
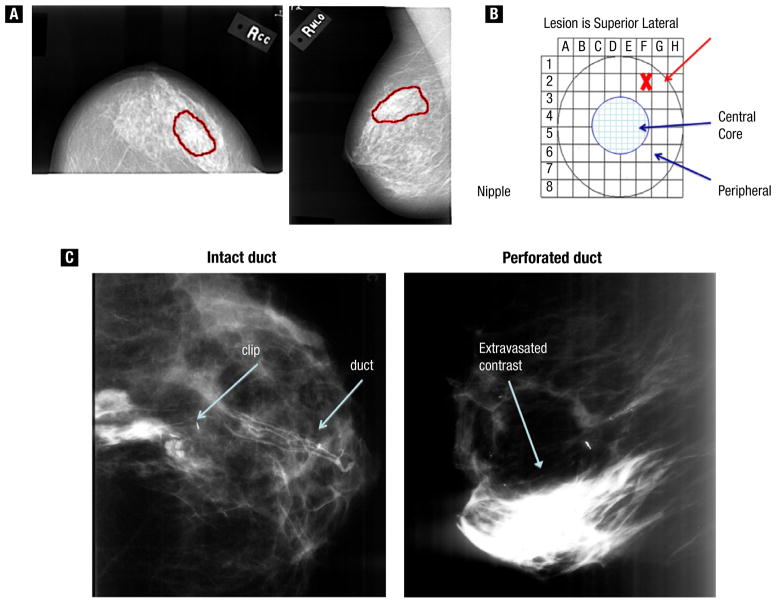
Based on preclinical data and previous clinical experience, we elected to use PLD, also known as Doxil Jansen, Horsham, PA, as the drug for intraductal delivery in this study. PLD is formulated to improve stability of the drug in the circulation,28 which may also help retain the drug in the ductal system of the treated duct.25 Of the 13 women enrolled in the study (Figure 1, for a schematic of the study design), 5 were treated with 20 mg PLD, one was treated with 4 mg PLD, one was treated with saline solution, 3 had duct perforations, and 3 had ducts that were either not found or not able to be cannulated (Table 1). We referred to the patients’ mammograms to identify the affected duct (Figure 2A, B) and also used ductography during the procedure to confirm that we had cannulated the correct duct and that it was intact (Figure 2C). In the case with the lower dose, the ductogram was ambiguous, and we stopped at 4 mg when the patient experienced pain. We subsequently determined that we erroneously cannulated an adjacent duct. Notably, the perforation rate in this study was approximately 25%, which is higher than the approximately 10% that we observed in cannulation of normal breast ducts, and may be because the ductal wall may be weakened in this disease. Adverse effects included 2 cases of localized erythema, one at 24 hours and one at 3 weeks, and 1 episode of delayed slight tenderness, none of which required treatment. To date, all the women have completed more than a year of follow-up without severe adverse events.
Figure 1.
Schematic of the Study Design
Table 1.
Intraductal Administration of Pegylated Liposomal Doxorubicin (PLD) Into Ductal Carcinoma In Situ Duct (DCIS) Containing Ducts
| Subject No. | Cannulation | Dose Doxil | Adverse Effects |
|---|---|---|---|
| 001 | Technical difficulties | — | — |
| 002 | Correct duct | 20 mg | Tenderness and erythema (d 23) |
| 003 | Adjacent duct | 4 mg | — |
| 004 | Duct not found | N/A | N/A |
| 005 | Perforated | N/A | N/A |
| 006 | Correct duct | 20 mg | None |
| 007 | Correct duct | 20 mg | Tenderness, erythema (24 h) |
| 008 | Perforated | N/A | N/A |
| 009 | Correct duct | 20 mg | Slight tenderness d 10–20 |
| 010 | Perforated | N/A | N/A |
| 011 | Correct duct | 20 mg | None |
| 012 | Could not cannulate | N/A | N/A |
| 013 | Correct duct | Saline solution | None |
Abbreviation: N/A = not applicable.
Figure 2.
Ductal Carcinoma In Situ Duct (DCIS) Identification. (A) Technique for Locating Nipple Opening That Corresponds to the Area on Mammogram. (B) Grid That Represents the Nipple Surface. (C) Ductogram, Showing Contrast Agent Localized to Within the Duct (left) or Leaking From the Perforated Duct into the Breast and Stroma (right)
MRI = magnetic resonance imaging; PLD = pegylated liposomal doxorubicin.
Histopathologic and Biomarker Analysis
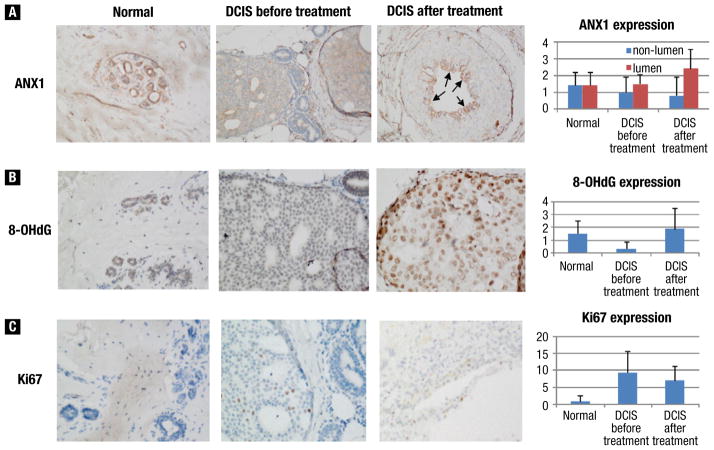
Tissue samples were available from a total of 7 individual patients, 6 of whom had samples from both before and after treatment. Of these 13 samples, 8 had 2 blocks, one from a DCIS-containing region and one from a non-DCIS area, that were used for immunohistochemical analysis of ANX1, 8-OHdG, and Ki-67. In our analysis of ANX1, we found that some DCIS areas had reduced ANX1 expression but that others had higher expression, which was mostly seen in epithelial cells toward the center or lumen of DCIS (Figure 3A). In general, ANX1 appeared to be reduced in DCIS areas overall but elevated in the lumen of DCIS after the treatment relative to the control. Similarly, we found that 8-OHdG expression was reduced in DCIS relative to normal tissue, although it appeared to increase after drug treatment (Figure 3B). In contrast, we found that Ki-67 expression was increased in DCIS relative to normal regions, as one would expect, and was slightly decreased in DCIS areas after treatment (Figure 3C). None of these differences reached statistical significance, however, due to the small sample size.
Figure 3.
Representative Images of Biomarker Expression in Normal (original magnification ×20), Ductal Carcinoma In Situ (DCIS) Before (original magnification ×40), and DCIS After Treatment (original magnification ×40). Immunohistochemical Stains for (A) Annexin-1 (ANX1), (B) 8-Hydroxydeoxyguanosine (8-OHdG), and (C) Ki-67. Bar Figures Show the Mean (standard deviation) of Staining Intensity (0, 1, 2, 3 for ANX1 and 8-OHdG) and Percentage of Positive Cells (for Ki-67) of All Cases Reviewed. Note for ANX1, Stain intensities in the Lumen and Nonlumen Areas Were Scored Separately
MRI Analysis
MRI data were received for a total of 7 patients: 4 had baseline (pretreatment) and posttreatment examinations that were analyzable; 2 had baseline and posttreatment examinations, but only the baseline examination was analyzable; and one had only a baseline examination. Nonanalyzable examinations were due to incomplete dynamic contrast-enhanced data. The 4 patients with MRI examinations at both pretreatment and posttreatment time points were evaluated for change in size and contrast kinetics. The presence of image artifacts in all the studies limited the ability to assess the extent of DCIS lesions. Differences in size measured pre- and posttreatment were not thought to be significant. None of the DCIS lesions demonstrated strong contrast kinetics generally associated with invasive disease. Of the 4 patients with paired MRIs, changes measured between visit 1 and visit 2 were not thought to be significant.
Discussion
Extensive preclinical data and 2 recent clinical trials demonstrated the safety and feasibility of instilling cancer drugs directly into the breast ducts through the nipple orifices.22,25 The utility of this approach, however, is more likely to be in premalignant disease. Although invasive cancer often destroys the ductal architecture and creates an obvious barrier to intraductal delivery of drugs, DCIS is by definition limited to an intact ductal system and, therefore, is uniquely suited to this therapeutic strategy. This study was designed to determine whether we could reliably identify the DCIS-containing duct, and to demonstrate that it is possible to deliver treatment throughout a diseased duct.
Ductal cannulation is routinely done clinically to investigate spontaneous nipple discharge by using ductograms. The involved duct in that situation is obvious and often dilated by the discharge, which facilitates cannulation. This study looked at whether we could identify the duct involved in previously biopsied DCIS and document it. By using mammography and a knowledge of duct anatomy, we were able to successfully identify and cannulate the DCIS-containing duct in 6 of the 13 subjects.
In 1 previous clinical study of intraductal therapy, Stearns et al22 had cannulated the most visible ductal orifice without effort to find the duct that led to the cancer. Our previous study of intraductal therapy was focused on demonstrating the feasibility of intraductal therapy as a chemoprevention strategy and involved cannulating as many ductal orifices as possible (5–8), again without attention to the particular duct involved in disease.25 The study presented here shows that the duct with DCIS can be identified and cannulated. Although, in this initial study, our success rate was just under 50%, it is probable that this would increase with more experience. This study also demonstrates the longest interval between intraductal therapy with PLD and surgery at 4–6 weeks. The absence of significant local or systemic adverse effects is significant because it suggests that local delivery can be administered safely.
Ductography at the time of the procedure was done both to document the duct as well as to look for perforation. In a previous study, sonoductography performed with more than 50 healthy women undergoing ductal lavage demonstrated a perforation rate of 11% with the currently available catheter.27 The increased perforation rate of 23% observed in our current study could be due to DCIS-containing ducts being more fragile. Alternatively, the additional pressure from a duct filled with DCIS could lead to perforation.
In an attempt to identify the effect of the therapy, we analyzed the expression of 3 biomarkers: ANX1, 8-OHdG, and Ki-67. The inflammatory marker ANX1, which is normally expressed in normal breast ductal epithelial cells, is typically lost or decreased in various types of precancerous lesions, including DCIS.29 Interestingly, we found that some DCIS areas had reduced ANX1 expression whereas others had higher expression, and ANX1 expression was increased in the lumen of DCIS after the treatment relative to control, which may reflect the response of malignant epithelial cells toward drug treatment. In addition, results of numerous studies found elevated levels of the oxidative stress marker 8-OHdG in various tumor tissues, including breast,30 colorec-tal,31 and lung,32 compared with healthy tissue, which supports a link between this type of oxidative damage and cancer development. Although somewhat counterintuitive, our findings that 8-OHdG expression is reduced in DCIS relative to normal tissue but increases after drug treatment agree with results of more recent studies that examined 8-OHdG expression in breast cancer tissue.33,34 Finally, high Ki-67 expression levels have been associated with poorer survival outcomes in breast cancer patients,35 and the protein has also been found to predict response to chemotherapy.36 Our findings that expression of the proliferation marker Ki-67 was increased in DCIS relative to normal regions and slightly diminished in DCIS areas posttreatment are not unexpected given the role of Ki-67 in cell proliferation.
Because we did not expect a change in microcalcifications, we explored the ability of MRI to detect changes as a result of intraductal therapy. Unfortunately, MRI did not distinguish any differences in the tissue before and after treatment. This may be because there were no changes in the extent of disease. Although MRI is extensively used in the breast, it has not been shown to reliably demonstrate the extent of DCIS and so may not be the best approach.
Conclusion
Overall, this study demonstrates the feasibility of cannulating ducts with DCIS and instilling therapy. Although recent preclinical evidence suggests that PLD may not be an appropriate agent,26 in-traductal therapy could be attempted with other drugs likely to have a local effect on DCIS, such as Herceptin (Genetach, South San Francisco, CA), curcumin,37 or tamoxifen. Difficulties in the ability to document the extent of DCIS as well as to monitor it over time inherently limit our capacity to manage this condition, but intraductal therapy is an attractive stepping-stone toward the development of improved DCIS treatment options.
Clinical Practice Points.
DCIS represent precancerous disease that is localized to one ductal system in the breast
It is possible to identify the appropriate duct and cannulate it
Treatment can safely be instilled into the duct
This may represent a potential for localized treatment of DCIS in the future
Acknowledgments
We thank Neil McDonald, Mary Langley, Marian Nelson, Brenda Shaw, Marie Sorci, Kelley Devlin-Lake, and Lydia Leonardo as well as the staff of St Joseph Hospital and the women who volunteered for this study. We are grateful to the California Breast Cancer Research Program for funding this project, grant no. 13OB-0185.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Holland R, Hendriks JH. Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol. 1994;11:181–92. [PubMed] [Google Scholar]
- 2.Wellings SR. A hypothesis of the origin of human breast cancer from the terminal ductal lobular unit. Pathol Res Pract. 1980;166:515–35. doi: 10.1016/S0344-0338(80)80248-2. [DOI] [PubMed] [Google Scholar]
- 3.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. 2010;2010:134–8. doi: 10.1093/jncimonographs/lgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–41. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 5.Lee RJ, Vallow LA, McLaughlin SA, et al. Ductal carcinoma in situ of the breast. Int J Surg Oncol. 2012;2012:1–12. doi: 10.1155/2012/123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96:906–20. doi: 10.1093/jnci/djh164. [DOI] [PubMed] [Google Scholar]
- 7.Holland R, Hendriks JH, Vebeek AL, et al. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990;335:519–22. doi: 10.1016/0140-6736(90)90747-s. [DOI] [PubMed] [Google Scholar]
- 8.Betsill WL, Jr, Rosen PP, Lieberman PH, et al. Intraductal carcinoma. Long-term follow-up after treatment by biopsy alone. JAMA. 1978;239:1863–7. doi: 10.1001/jama.239.18.1863. [DOI] [PubMed] [Google Scholar]
- 9.Eusebi V, Feudale E, Foschini MP, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11:223–35. [PubMed] [Google Scholar]
- 10.Page DL, Dupont WD, Rogers LW, et al. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer. 1982;49:751–8. doi: 10.1002/1097-0142(19820215)49:4<751::aid-cncr2820490426>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Sanders ME, Schuyler PA, Dupont WD, et al. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–4. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 12.Erbas B, Provenzano E, Armes J, et al. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–44. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 13.Tot T. DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch. 2005;447:1–8. doi: 10.1007/s00428-005-1274-7. [DOI] [PubMed] [Google Scholar]
- 14.Mai KT, Perkins DG, Mirsky D. Location and extent of positive resection margins and ductal carcinoma in situ in lumpectomy specimens of ductal breast carcinoma examined with a microscopic three-dimensional view. Breast J. 2003;9:33–8. doi: 10.1046/j.1524-4741.2003.09108.x. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the national surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–18. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs L, Sukumar S, Stearns V. Intraductal therapy for the prevention of breast cancer. Curr Opin Investig Drugs. 2010;11:646–52. [PubMed] [Google Scholar]
- 17.Flanagan M, Love S, Hwang ES. Status of intraductal therapy for ductal carcinoma in situ. Curr Breast Cancer Rep. 2010;2:75–82. doi: 10.1007/s12609-010-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z, Shen T, Wientjes MG, et al. Intravesical treatments of bladder cancer: review. Pharm Res. 2008;25:1500–10. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Echarri Gonzalez MJ, Green R, Muggia FM. Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncol Williston Park. 2011;25:156–65. 170. [PubMed] [Google Scholar]
- 20.Okugawa H, Yamamoto D, Uemura Y, et al. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Res Treat. 2005;91:29–34. doi: 10.1007/s10549-004-6455-6. [DOI] [PubMed] [Google Scholar]
- 21.Murata S, Kominsky SL, Vali M, et al. Ductal access for prevention and therapy of mammary tumors. Cancer Res. 2006;66:638–45. doi: 10.1158/0008-5472.CAN-05-4329. [DOI] [PubMed] [Google Scholar]
- 22.Stearns V, Mori T, Jacobs LK, et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci Transl Med. 2011;3:106–8. doi: 10.1126/scitranslmed.3002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love SM, Barsky SH. Anatomy of the nipple and breast ducts revisited. Cancer. 2004;101:1947–57. doi: 10.1002/cncr.20559. [DOI] [PubMed] [Google Scholar]
- 24.Goulet RJ, Badve S, Brannon-Peppas L, et al. Pilot trial assessing the feasibility of intraductal delivery of epirubicin (epi)-containing nanoparticles (NP) via induct breast microcatheter (IDBM) Proc Am Soc Clin Oncol. 2004;22:828a. (abstract) [Google Scholar]
- 25.Love S, Zhang B, Gordon EJ, et al. A feasibility study of the intraductal administration of chemotherapy. Cancer Prev Res (Phil) 2013;6(1):51–8. doi: 10.1158/1940-6207.CAPR-12-0228. [DOI] [PubMed] [Google Scholar]
- 26.Chun YS, Yoshida T, Mori T, et al. Intraductally administered pegylated liposomal doxorubicin reduces mammary stem cell function in the mammary gland but in the long term, induces malignant tumors. Breast Cancer Res Treat. 2012;135:201–8. doi: 10.1007/s10549-012-2138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tondre J, Nejad M, Casano A, et al. Technical enhancements to breast ductal lavage. Ann Surg Oncol. 2008;15:2734–8. doi: 10.1245/s10434-008-0060-6. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W, Lionberger R, Yu LX. In vitro and in vivo characterizations of pegylated liposomal doxorubicin. Bioanalysis. 2011;3:333–44. doi: 10.4155/bio.10.204. [DOI] [PubMed] [Google Scholar]
- 29.Shen D, Nooraie F, Elshimali Y, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–91. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Matsui A, Ikeda T, Enomoto K, et al. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87–95. doi: 10.1016/s0304-3835(99)00424-3. [DOI] [PubMed] [Google Scholar]
- 31.Oliva MR, Ripoll F, Muñiz P, et al. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol Carcinog. 1997;18:232–43. [PubMed] [Google Scholar]
- 32.Shen J, Deininger P, Hunt JD, et al. 8-hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007;109:574–80. doi: 10.1002/cncr.22417. [DOI] [PubMed] [Google Scholar]
- 33.Karihtala P, Kauppila S, Puistola U, et al. Divergent behaviour of oxidative stress markers 8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxy-2-nonenal (HNE) in breast carcinogenesis. Histopathology. 2011;58:854–62. doi: 10.1111/j.1365-2559.2011.03835.x. [DOI] [PubMed] [Google Scholar]
- 34.Sova H, Jukkola-Vuorinen A, Puistola U, et al. 8-hydroxydeoxyguanosine: a new potential independent prognostic factor in breast cancer. Br J Cancer. 2010;102:1018–23. doi: 10.1038/sj.bjc.6605565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17:R245–62. doi: 10.1677/ERC-10-0136. [DOI] [PubMed] [Google Scholar]
- 36.Chang J, Ormerod M, Powles TJ, et al. Apoptosis and proliferation as predictors of chemotherapy response in patients with breast carcinoma. Cancer. 2000;89:2145–52. [PubMed] [Google Scholar]
- 37.Chun YS, Bisht S, Chenna V, et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 2012;33:2242–9. doi: 10.1093/carcin/bgs248. [DOI] [PMC free article] [PubMed] [Google Scholar]