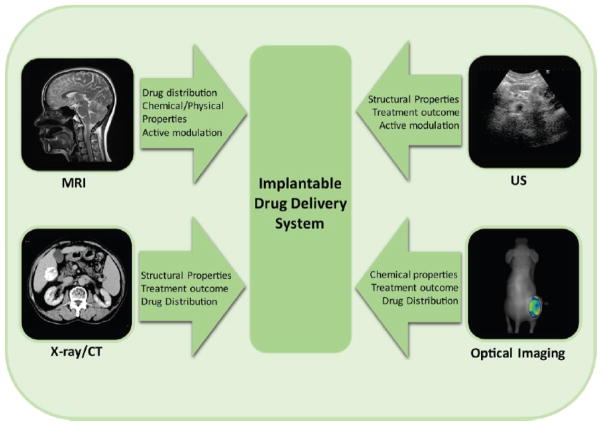
Abstract
Implantable drug delivery systems (DDS) provide a platform for sustained release of therapeutic agents over a period of weeks to months and sometimes years. Such strategies are typically used clinically to increase patient compliance by replacing frequent administration of drugs such as contraceptives and hormones to maintain plasma concentration within the therapeutic window. Implantable or injectable systems have also been investigated as a means of local drug administration which favors high drug concentration at a site of interest, such as a tumor, while reducing systemic drug exposure to minimize unwanted side effects. Significant advances in the field of local DDS have led to increasingly sophisticated technology with new challenges including quantification of local and systemic pharmacokinetics and implant-body interactions. Because many of these sought-after parameters are highly dependent on the tissue properties at the implantation site, and rarely represented adequately with in vitro models, new nondestructive techniques that can be used to study implants in situ are highly desirable. Versatile imaging tools can meet this need and provide quantitative data on morphological and functional aspects of implantable systems. The focus of this review article is an overview of current biomedical imaging techniques, including magnetic resonance imaging (MRI), ultrasound imaging, optical imaging, X-ray and computed tomography (CT), and their application in evaluation of implantable DDS.
Keywords: Biomaterials, drug delivery, fluorescence imaging, implant, MRI (magnetic resonance imaging), scaffold, ultrasound imaging, X-ray CT Imaging
INTRODUCTION
Traditional routes of administration, which include oral, topical or intravenous injections, result in immediate or expedited release and bioavailability of the active agent. This bolus of drug often causes a spike in plasma concentration of drug temporarily above the therapeutic window and requires frequent administration in order to maintain therapeutic efficacy. This frequent administration of drug not only causes variable drug concentrations, but can make it difficult for patients to maintain the proper regimen. Ideally, the concentration of drug should remain constant within the therapeutic window while requiring minimal administration in order to achieve the desired treatment efficacy. Implantable polymeric DDS have been studied extensively over the last few decades as a means to achieve extended therapeutic concentrations of drugs for various diseases [1–5]. The motivation behind the development of these systems can be attributed to benefits such as longevity, predictable steady state pharmacokinetics, decreased frequency of administration and improved patient compliance [6–8]. One of the earliest examples of an implantable drug delivery device was Norplant®, a subcutaneous implant made from nondegradable crosslinked polydimethylsiloxane (PDMS), for the extended release of a contraceptive steroid [9]. In 1990, Norplant® received FDA approval and by 1992 was utilized by over 600,000 women in the United States [10, 11]. At the time, Norplant® was considered one of the most effective in preventing pregnancy for a longer period of time than any other available contraceptive. Although relatively successful, complications sometimes arose due to the long implantation period, which allowed for fibrous encapsulation of the implants and made them difficult to remove. Incomplete removal was common due to implant fracture and often involved ultrasound imaging to locate the residual implant [12].
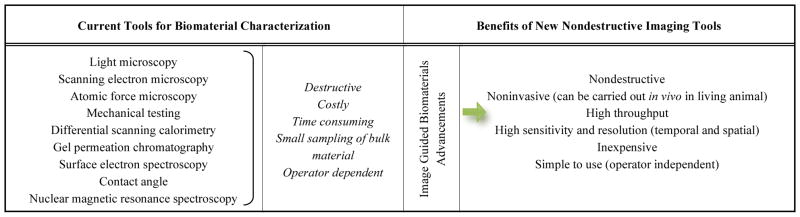
Other early implantable drug delivery vehicles similar to Norplant® all involved incorporating a drug in a nondegradable polymer matrix. Polymers such as poly(ethylene-co-vinyl acetate) (polyEVA) or crosslinked PDMS, were initially used because they were relatively biologically inert and biocompatible. However, as with Norplant®, these implants required invasive and sometime difficult removal procedures [7, 12]. This resulted in a shift to the use of biodegradable polymers, such as polyanhydrides or polyesters, for implantable DDS. Although there has been some clinical success with these types of materials - Lupron Depot [13], Eligard [14], or Gliadel wafer [15] are good examples - the majority of innovative implantable technologies have remained in the preclinical phase due to challenges with clinical translation. Notably, while these devices are able to concentrate therapeutic agents at the target site (e.g. a tumor), their eventual in vivo behavior is unpredictable because it is typically governed by uncontrollable physiological, biological and chemical processes of the surrounding tissue. These processes result in poorly understood in vivo implant behavior and, subsequently, a deficient in vitro-in vivo correlation hampering implant research, development and clinical translation. Reliable tools that are able to gather the necessary information about implant behavior in vivo are not readily available, yet quantifying factors that impact implant formation, degradation, and local drug distribution is essential to improving their performance. Because the FDA requires characterization of implant properties such as biodegradation, interface characteristics with tissue, and release kinetics etc., prior to clinical trials, translational bottleneck has been created, especially in strategies incorporating various biological, chemical and mechanical components that can increase the cost and time required to complete the product development pipeline. It is currently estimated that the entire FDA approval process takes between 10–15 years and costs about 1 billion dollars [16–20]. Currently, high throughput methodologies are not commonplace in the fabrication and evaluation of implantable DDS, particularly in vivo. Instead, studies to investigate and comprehensively determine in situ forming implant (ISFI) properties are costly and inefficient and utilize tools and techniques (summarized in Table 1) that are destructive and highly operator dependent.
Table 1.
Comparison of current techniques and proposed tools in characterization of biomaterials
|
It thus stands to reason that the streamlined, efficient evaluation of material or medical device performance in vivo is now more than ever of paramount importance to the advancement of the drug delivery field. In vivo animal work is expensive, time consuming, cumbersome (as current strategies are typically combined with ex vivo analysis), and ethically questioned in many circles. In vitro and mathematical models of in vivo systems are potentially intriguing, but it is unlikely that any one of such systems can account for the multitude of complex processes taking place at any given time in vivo. In contrast, medical imaging technology can provide the tools to replace traditional characterization techniques and revolutionize the way we advance the development of biodegradable implant technologies. Biomedical imaging techniques offer the possibility of high throughput, longitudinal measurements and analysis of devices in their intended implanted state. The great variability in the energy sources of different imaging modalities offers a diverse set of imaging depths (0.3mm to fully body) and contrast and spatial resolutions (0.2–200um) that can be applied to the characterization of implants regardless of their composition or site of placement (Table 2). Of the many imaging modalities available, four have been specifically and extensively used to characterize implantable DDS and include ultrasound imaging, MRI, optical imaging, and radiographic imaging. This review paper serves to provide insights on the biomedical imaging modalities available based on the advantages and limitations associated with their ability to characterize the various aspects implantable DDS.
Table 2.
| Imaging Modalities | Spatial Resolution | Real Time | Penetration Depth | Drug Tracking Capability | Portability | Cost |
|---|---|---|---|---|---|---|
| Micro-US | 20–100μm | Yes | 10mm | No | High | Low |
| Micro-MRI | 200μm | No | Full body | Yes | Low | High |
| Fluorescence microscopy | 0.2–1μm | Yes | 0.3–1.0mm | Yes | High | Low |
| OCT | 1–15μm | Yes | 1–3mm | No | High | Low |
| Micro-CT | 5μm | No | Full body | Yes | Low | Medium |
US= ultrasound; MRI= magnetic resonance imaging; OCT= optical coherence tomography; CT= computed tomography
ULTRASOUND
In medical imaging, ultrasound utilizes the propagation of sound waves ranging from 2–15 MHz in organs and tissues of different acoustic impedance to produce anatomical images. This is implemented by using an ultrasound transducer, which is capable of both producing and receiving sound waves. When these sound waves travel through a medium, such as tissue, a portion of the sound waves is reflected back to the transducer as they interact at the interfaces of materials with different acoustic impedances. The depth of the imaging structure is determined by the time between firing the ultrasound signal and receiving the echo. The amplitude of the echo, or portion of reflected sound waves, is encoded and displayed in a gray-scale image. Acoustic impedance, governed by the density and bulk modulus of a material, determines the ultrasound image contrast. This mechanism allows ultrasound to provide structural/mechanical information about an implantable DDS. In combination with its noninvasive nature and high temporal resolution (up to 500 frames per second), ultrasound has been used to continuously monitor implant properties such as positioning within the body [28–30], morphology [28, 31–33], and associated tissue response in vivo [31, 34]. In early work, ultrasound was extensively used for the placement, extraction and characterization of implants for the sustained delivery of contraceptive steroids [35, 36]. In cases where intrauterine devices have been left in the uterus for long periods of time, ultrasound was also used as a method to detect fragmented implants that can cause complications [34]. In another study, ultrasound was used in prostate brachytherapy to guide the placement of 125I containing implants and visualize the implants distribution in real-time [37].
Upon implantation of a foreign biomaterial, it is also important to be able to noninvasively monitor the host response to the material over time. The reaction can lead to implant damage and hampering of the long term performance. Changes in tissue architecture surrounding implants inserted in both the eye [31] and the vasculature [38, 39] have been monitored using ultrasound as a measurement of biocompatibility or treatment outcome. Serruys et al. used quantitative intravascular ultrasound (IVUS) to evaluate the treatment outcomes of their anti-proliferative drug-eluting vascular scaffolds by quantifying the area of obstruction in the lumen [38]. In another study by Reibaldi et al, a 50 MHz ultrasound biomicroscope was used to evaluate a hyaluronan based intravitreal implant for sustained release of antiproliferative drugs. Here, the internal ultrasound reflectivity of the implant and the surrounding tissue response, which correlates to implant biodegradation and biocompatibility respectively, were monitored for 150 days in vivo [31]. In the context of cell delivery using polymeric scaffolds, ultrasound has also been used to quantitatively evaluate bone marrow stromal cell numbers in a β-Tricalcium phosphate composites by correlating the cell density to ultrasound amplitude [40].
With the advent of polymer implants that form in situ rather than traditional preformed implants, there has been a strong need for ways to noninvasively characterize their behavior in vivo. The transition from a liquid to solid depot requires an imaging technique that has both high spatial and temporal resolution, as this transition step is critical to predicting drug release kinetics [33, 41, 42]. In 2009, Solorio et al. developed a technique using ultrasound b-mode imaging and grayscale analysis to monitor the phase inversion kinetics of in situ forming PLGA implants and correlated the process with in vivo drug release [32]. The phase transition process from liquid to solid causes a dramatic change in acoustic impedance which can be detected by ultrasound in real time. A validation study was performed in our lab by comparing ultrasound b-mode images to digital photos of the same implant (Fig. 1A, B).
Fig. 1.
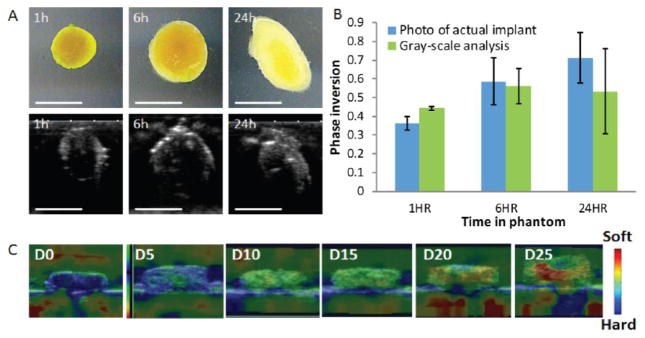
Examples of ultrasound in implantable DDS. (A) Representative gray-scale images of the implants over time below photos of the actual implant. Scale bar is 5mm (B) Quantification of phase inversion calculated using photos of actual implants and ultrasound gray-scale analysis. (C) Representative ultrasound elastography images of implants over time indicating implants becoming soft. (Unpublished data).
Information on in vivo implant erosion and biodegradation is critical when designing degradable DDS. Methods for measuring degradation and erosion such as gravimetrical analysis and gel permeation chromatography (GPC) are destructive and time-consuming, leaving a need for a less invasive way to monitor polymer behavior. Many have investigated using ultrasound to assess polymer degradation and erosion of tissue scaffolds through the use of a special technique called ultrasound elastography (UE) [43, 44]. Ultrasound elastography is a dynamic technique that uses ultrasound to assess the mechanical stiffness of materials noninvasively by measuring material distortion or strain in response to external compression. In our lab, we have tested the feasibility of using implant mechanical properties measured by UE to estimate its rate of polymer erosion (unpublished data). In this study, in situ forming PLGA implants with various molecular weights (17kDa, 34kDa and 52kDa) were injected into a tissue mimicking phantom. UE scans were performed daily and compared to erosion measurements gathered by gravimetrical analysis. By comparing the UE data to the erosion data, we observed that the rate of change in mechanical properties correlates well with the rate of implant erosion. Representative ultrasound elastography images shown in Fig. (1C), indicate the implant softening process overtime due to polymer degradation and erosion.
Applications of ultrasound for characterization of implantable DDS have not been limited to imaging of their physical structures. Increasing the power of ultrasound well beyond diagnostic levels has been investigated as an external way to modulate drug release from an implantable DDS. This capability of ultrasound was attributed to the cavitation and acoustic streaming associated with the increased power [45, 46], but the exact mechanism has not yet been fully understood. The first attempt was done in 1985 by Miyazaki et al. using ultrasound to increase drug release from an ethylene-vinyl alcohol copolymer [47]. Then Kost et al. tested the feasibility of ultrasound to modulate drug release from both biodegradable [46] and non-degradable [48] polymeric delivery systems. In this study, up to a 5-fold reversible increase in degradation rate and up to a 20-fold reversible increase in release rate was observed in implants made from polyanhydrides, polyglycolides and polylactides. In addition to modifying the drug release from implantable systems, ultrasound at various frequencies has also been applied to enhance transdermal transport of low molecular weight drugs and proteins [49].
MAGNETIC RESONANCE IMAGING (MRI)
Magnetic resonance imaging (MRI) is a non-invasive imaging technique that depends on both the local proton density and the molecular environment to generate 2-dimensional (2D) anatomical images. MR images are produced by applying a strong magnetic field (1.5–11.7 Tesla) to a sample, roughly aligning a number of protons with the direction of the field, which can be considered as a magnetization vector. These protons experience a torque and precess around the field axis at the Larmor frequency specific to the magnetic field of the scanner [50]. Radiofrequency pulses are applied to modify the magnetization vector, and as the vector relaxes back to its alignment, it emits this energy which can then be measured using coils. Different molecular environments influence the behavior of the magnetization, which in turn influences the MR signal. Various pulse sequences that cause differences in the magnetization due to T1 and T2 relaxation time and proton density can be used to selectively create MR images with varying contrast [50].
Unlike with other imaging modalities, the measurement of the time constants, T1 and T2, in MR allows us to not only investigate the physical properties, but also the chemical properties of materials. For this reason MRI has been increasingly used for characterization of implantable DDS both in vitro and in vivo [51–53]. Implant properties, such as water uptake [54], spatial variation in matrix degradation [55], and their correlation to release kinetics [56] have been obtained with MRI. In one study, MRI was used to examine the buffer uptake kinetics into a PLGA implant after incorporating a high molecular weight peptide by correlating liquid concentration to T1/T2 contrast maps. A strong peptide-polymer interaction was observed which affected the polymer chain configuration and mobility, thus changing the water uptake kinetics [54]. All of these examples however, rely on relative changes in the contrast generated by MRI scanners and thus have been limited by the mostly qualitative nature of this imaging modality. Ma et al. have recently developed a new approach in the acquisition and postprocessing of magnetic resonance signals, termed magnetic resonance fingerprinting (MRF), which performs a quantitative analysis on the MR signal that is based on physical and chemical changes within materials and tissues [57]. This new technique has the potential to allow for MRF to noninvasively monitor complex changes in implantable DDS such as degradation, chemical activity, tissue response, etc. Although current MRI systems have several advantages over other imaging modalities, the high installation and running cost of clinical MRI has prevented its further use in the drug delivery community. At the cost of a smaller bore, a bench-top MRI (BT-MRI), which has similar capabilities as clinical MRI, is a cost effective alternative and is more ideal for pre-clinical imaging of implantable devices. Mader et al. used BT-MRI and electron paramagnetic resonance (EPR) spectroscopy to non-invasively monitor injectable ISFI behaviors including solvent exchange, precipitation and degradation in vitro and in vivo [41, 57].
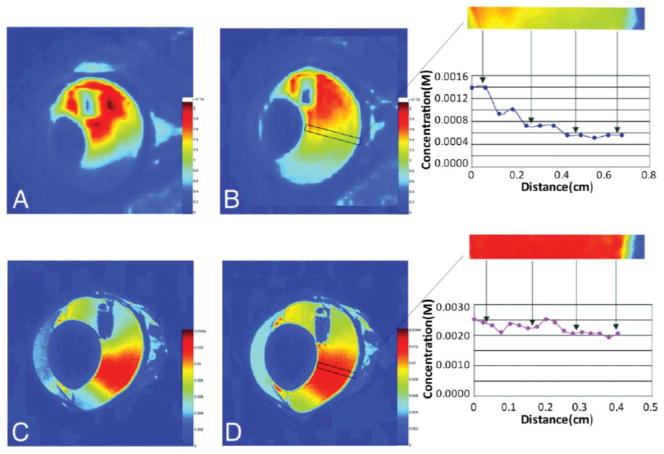
The use of MR contrast agents, such as gadolinium or iron oxide-based agents, enables MRI to measure drug distributions throughout the body. Gadolinium based contrast agents have been used as mock drugs to study the distribution of drug away from implant systems in targeted tissues such as the eye [58, 59], brain [60], bone [61–63] and muscle [63, 64]. In one study by Csaky et al., the mass transport and distribution of an ocular drug released from a polyvinyl alcohol (PVA) polymeric implant was investigated by using its surrogate, Gd-DTPA (Fig. 2) [58]. In another study with Gd-DTPA, which is similar in size and diffusion coefficient to the antimicrobials gentamicin and vancomycin, was also used to visualize drug transport from orthopedic implants [65]. In this study, implants of different materials were implanted in either the intramedullary canal or the quadriceps and the local drug distubtions away from the implants were assessed using MRI. The implantation site was shown to have a dramatic effect on the distribution and movement of drug [63].
Fig. 2.
Representative MRI images of an intravitreal implant. Concentration of Gd-DTPA is correlated to a color scale bar on the right of each image. (A, B) In vivo images of 4 and 7 hours after implantation respectively, indicating the distribution of Gd-DTPA in the vitreous cavity. The boxed area of vitreous is magnified, and the concentration of Gd-DTPA over distance is quantified on the right. (C, D) Ex vivo image of 4 and 7 hours after implantation respectively, indicating the distribution of Gd-DTPA in the vitreous cavity. The boxed area of vitreous is magnified, and the concentration of Gd-DTPA over distance is quantified on the right. (Adapted from Ref. [58], with permission).
In contrast to gadolinium, iron oxide based contrast agents have mainly been used in the context of cell delivery using polymeric scaffolds. Contrast agents including Super-Paramagnetic Iron Oxide (SPIO), and Monocrystalline Iron Oxide Nanocompounds (MION) produce low intensity in MR images. These contrast agents are more biocompatible than gadolinium allowing their use as cell labeling agents [66]. They are typically incorporated into a delivered cell enabling MRI tracking of the distribution and concentration of these cells [67]. Bouzier-Sore et al have used MRI to track the SPIO labeled human Adipose Derived Stem Cells from a porous polysaccharide based scaffold for bone regeneration [66]. SPIO has also been used by Hoehn et al. to monitor the embryonic stem cell migration after implantation into brain and was validated by using green fluorescent protein (GFP) registration [68].
The power of magnetic fields is not limited to the imaging of polymeric materials, but can be used to modulate drug release from DDS. By exciting magnetic microspheres with applied magnetic fields, drug release from an implantable DDS can be controlled. It has been shown that the drug release from a magnetite embedded polymer matrix can be triggered or increased by applying oscillating magnetic fields [69–72]. This finding was attributed to the movement of the iron beads due to the oscillating magnetic field, which produced “micro-cracks” in the matrix, thus facilitating the influx of liquid and efflux of drug [70]. In vivo studies by Kost et al. showed that subcutaneously implanted polymer matrix containing insulin and magnetic beads can repeatedly reduce glucose levels on demand by applying oscillating magnetic field [71]. The effect of various magnetic field amplitudes [70] and frequencies [72] on drug release from the magnetically excitable DDS has also been investigated.
OPTICAL IMAGING AND OPTICAL COHERENCE TOMOGRAPHY (OCT)
Optical imaging techniques such as microscopy, fluorescence imaging, and optical coherence tomography have been used extensively in the development and characterization of DDS. Optical imaging measures the interactions of light with matter to produce 2-dimensional images with high spatial and temporal resolution. Advancements in detection of photons and highly specific biomarkers have allowed optical imaging to be at the forefront of molecular imaging at the cellular and subcellular level. However, the low energy source limits this imaging modality to mostly surface or thin sample characterization. For this reason the majority of optical imaging applications in implantable DDS involve invasive procedures such as the use of ex vivo resection of samples and cryosectioning. Nevertheless, the development of in vivo optical imaging techniques, such as OCT, have allowed scientists to non-invasively study implant characteristics in vivo in the preclinical setting.
In the development of implantable DDS for localized drug delivery, it is essential to have the capability of measuring both drug release kinetics, and drug distribution away from implants. These characteristics are essential to achieving effective drug concentrations within the appropriate treatment volume. Fluorescence imaging is widely used for this purpose on extracted tissues [73–75]. In this process, tissue is removed and cryosectioned into thin pieces followed by fluorescence imaging for drug detection. This technique benefits from high spatial resolution and low detection limit while suffering from the fact that very few drugs are fluorescent. Weinberg et al. used fluorescence imaging to measure the distribution of drug away from their polymer implants while implanted in a rabbit liver tumor model. In this study, doxorubicin, which has intrinsic fluorescence, was delivered using preformed PLGA millirods after a radiofrequency ablation. This study revealed that the drug distribution was highly dependent on tissue architecture (ablated or non-ablated tissue) [75]. As stated earlier, many drugs are not intrinsically fluorescent. To circumvent this drawback, drugs without natural fluorescent can be labeled with a fluorescent tag. However this labeling process may also change the drug structure, thus resulting in different therapeutic efficacy and transport properties.
In addition to the direct measurement of drug release and distribution, an understanding of the interactions between implants and their local physiological environment is equally important. Processes including polyester degradation and erosion through hydrolysis when implanted in the aqueous tissue environment [76], pH changes inside the in situ forming implants [77, 78] and infections as a result of tissue response to the implant system have all been studied using fluorescence imaging [79]. In order to use fluorescence as a measure of degradation of polymeric implants, a new type material called aliphatic biodegradable photoluminescent polymers (BPLPs) was developed. This polymer demonstrated great cytocompatibility in vitro, minimal chronic inflammatory response in vivo and tunable fluorescence emission [80]. Artzi at el. have also used fluorescence to monitor in vivo hydrogel erosion. In their study, a fluorescently tagged polymer was implanted in an animal model. The loss of fluorescence signal with time was converted to erosion. In vitro and in vivo erosion was found to be well correlated suggesting the possibility of using in vitro erosion data to predict in vivo erosion (Fig. 3) [76].
Fig. 3.
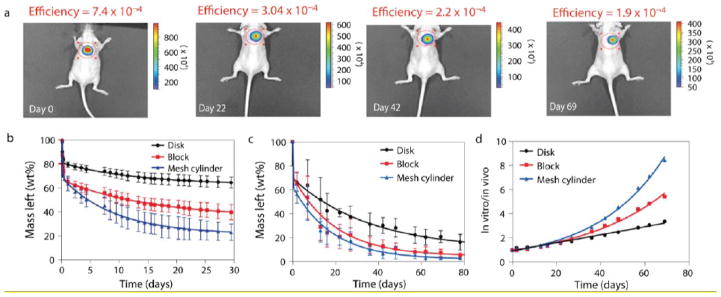
In vitro–in vivo erosion profiles of PEG: dextran correlate and vary with material surface area. In vitro and in vivo erosion profiles of PEG: dextran cast in a series of shapes are depicted by tracking the loss of fluorescence intensity with time. (A) The effect of material shape on degradation profile was followed in vivo non-invasively in the dorsal subcutaneous space of mice (disk shaped materials are presented). B–D, The loss of fluorescence signal with time in vitro (B) and in vivo (C) was converted to weight loss and correlated (D). Correlations found between mean values of in vitro and in vivo erosions are indicated. (Adapted from Ref. [76] with permission).
Optical coherence tomography (OCT) is an optical method analogous to ultrasound imaging, which uses the coherence between backscattered light from an object and reference light to synthesize 2D and 3D images. Owing to the precise measurement of distance using this coherence method and employment of long wavelength light (typically near-infrared light), OCT provides superb spatial resolution (<10 μm) and moderate penetration (1–2 mm), offering advantages over other imaging modalities. In the clinical setting, OCT has been primarily used to distinguish normal from pathological tissues in vivo and ex vivo. For this reason, OCT has been used to evaluate the treatment outcomes in studies involving drug eluting stents [81–84]. In a study by Guagliumi et al., OCT was used to study the vascular tissue response (neointimal tissue thickness and stent cover rate) from anti-proliferative agent eluting stents implanted in patients with long coronary stenosis.
In addition to measuring tissue response, OCT has also been used for other purposes such as visualizing the microstructure of the implant matrix [85, 86], quantifying the diffusion of drug in the eye [87] and guiding the placement of implants [88]. In one study, Patterson et al. used 3D OCT to characterize the in situ degradation of PLGA microspheres contained within a hyaluronic acid hydrogel gel after implantation into a rat skull defect model. The quantitative degradation was estimated by monitoring the change in average microsphere diameter. Furthermore, the obtained degradation rate was found to be correlated to the drug release from the implant [85]. OCT can also be used for quantitative measurements such as investigating the permeability of drug transport through tissues. A study by Ghosn et al. showed the feasibility of using OCT to nondestructively estimate the permeability coefficient of different small molecular weight drugs through rabbit cornea and sclera [87].
X-RAY IMAGING AND COMPUTED TOMOGRAPHY (CT)
When highly energetic electrons interact with matter, their kinetic energy is converted into electromagnetic radiation, producing X-rays. An X-ray beam interacts variably with tissues, being highly attenuated by bone compared to soft tissue, for example. This interaction produces variable X-ray transmission and results in a ‘shadow’ of the anatomical structures. The transmitted radiation is captured by an analog or, more frequently, a digital detector which then converts the X-rays into a 2D film or digital image. During the last 40 years, computed tomography (CT) has revolutionized X-ray imaging by using linear detector arrays for acquisition of multiple beam projections which are then converted into tomographic images which can later be reconstructed into 3D images, providing quantitative volumetric information. In medical diagnostic applications, high-energy photons (greater than 15 keV) are utilized because they are not easily absorbed through tissue, allowing for whole body imaging. The high use cost and relatively low resolution of diagnostic CT scanners (range from 0.5–1 mm) have limited their use in preclinical small animal studies. Advances in detector technology and computer processing has allowed for high-resolution micro-CT scanners that can achieve isometric voxel sizes as small as 5 um [27]. This improved spatial resolution has allowed scientists to noninvasively look at microstructural changes in polymeric DDS.
Micro-CT, with the exception of a few examples, has been used in drug delivery for imaging and analysis of the 3D structure and morphology of polymeric implants. Specifically, micro-CT has been used to look at in vivo structural properties of implants such as tortuosity [89, 90], porosity, average pore size [91–93] and their correlation to release kinetics [94, 95]. The resolution of CT has even allowed for 3D visualization of porosity changes in PLGA scaffolds with different concentrations of porogens [93]. Due to the high absorbing properties of bone, the use of radiographic imaging in the preclinical setting has been primarily used in the study of implants associated with bone tissue engineering. Micro-CT has been used to evaluate bone ingrowth into [96–98], or changes in bone morphology [99, 100] around drug/peptide eluting scaffolds. Although the majority of clinically available drugs provide poor contrast under X-ray, heavy metal drugs like cisplatin, a platinum based anticancer drug, have high X-ray attenuation and thus provide inherent contrast. Exner et al., have exploited this property and developed a non-invasive method using CT to measure both the drug release kinetics from and local drug concentrations surrounding their PLGA millirods [101–103]. Unlike optical fluorescent techniques, the use of CT to measure drug concentration allows for deep penetration distances with high spatial resolution allowing for monitoring in vivo in large animals.
Although the contrast between most biomaterials and tissue under radiographic imaging is relatively low, CT has been clinically useful in the treatment of hepatocellular carcinoma with transarterial chemoembolization (TACE). While not an implantable system, TACE is a loco-regional technique that relies solely on image guidance for its administration. During a TACE treatment, CT is used to guide a catheter while it is inserted into the local blood supply of a tumor. Once at the tumors feeding blood vessel, an embolic agent is released to block the blood flow, initiating ischemic tumor necrosis. Lipiodol, a commonly used embolic agent, made from an iodinated ethyl ester of fatty acids in poppy seed oil, has also been investigated for the controlled release chemotherapeutic drugs such as doxorubicin [104]. Nakamura et al. has shown that by adjusting the ratio of Lipiodol to doxorubicin, pharmacokinetic outcomes can be better than direct drug infusion. Also, because it is iodinated, Lipiodol acts as a CT contrast agent, allowing for non-invasive monitoring of tumor accumulation and distribution (Fig. 4) [105]. The degree of distribution within the tumor has been strongly correlated, through histopathology, to tumor necrosis [106].
Fig. 4.
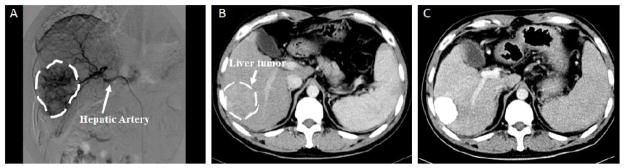
Pre- and peri-operative imaging for transarterial chemoembolization (A) Digital subtraction angiography image of hepatocellular carcinoma tumor and its corresponding feeding vessel branches of the hepatic artery. Noncontrast CT image showing tumor prior to TACE treatment (B) and post treatment accumulation of Lipiodol in tumor (C). All tumor regions are encircled by the dashed line. Images reproduced with permission from Dr. Yong Wang, Wu Han Union Hospital, China.
Even with benefits such has superior penetration and spatial resolution to other imaging modalities, the use of X-ray imaging also has several drawbacks. For one, X-ray radiation is ionizing, and thus can be harmful to patients if used frequently. The ionizing radiation is often a limiting factor when performing long term implant characterization studies that require frequent exposure in order to achieve high temporal resolution. In addition, X-ray imaging does not typically provide great contrast between different soft tissues and polymeric implants, making it necessary to coat the implants with contrast agents, such as Hexabrix, to distinguish the boundaries [107]. These coatings, if too thick, can limit CTs ability to look at surface structures.
CONCLUSION
Understanding implantable DDS in vivo behavior is essential for ensuring a smooth translation into the clinical setting. To accomplish this task, noninvasive, nondestructive analysis tools are quickly becoming an essential component of preclinical research in this field. Most important, as described above, are the various biomedical imaging modalities that can be applied to monitor specific aspects of implantable DDS including morphology, physiochemical properties, tissue response to the implant and therapeutic agent and drug distribution. Certainly, each imaging modality has its unique advantages and limitations; Fig. (5) summarizes the capabilities of each modality in applications for DDS. For example, optical imaging provides high spatial resolution and functional information through the use of various biomarkers while suffering from the lack of penetration depth, making it ideal for the in vitro or ex vivo applications. In contrast, CT offers a wide field of view, high temporal and spatial resolution and deeper penetration, yet it lacks contrast between different soft tissues. In order to obtain more comprehensive information, researchers are not limited to the use of a single imaging modality. Rather, the use of multimodal imaging can overcome the limitations of individual techniques while combing desired advantages. It is also important to look for ways to exploit the diverse energy sources associated with the different imaging modalities in order to not only image implantable systems but manipulate their behavior on demand. A constant rate of drug delivery may not be ideal in all clinical settings. For instance, patients with conditions such as diabetes, heart rhythm disorders, angina pectoris, etc. may need a pulsed or even programmable delivery pattern based on the stages of biological conditions. Magnetic and ultrasonic fields have already been used to externally manipulate drug release kinetics at will, but are still in their infantile stage. With proper development, they may be used to control drug release profile in a more precise manner. The beauty of nondestructive imaging analysis is that each implantable technology can be investigated with several techniques all in the same animal over the lifetime of the single device. This can significantly reduce inter-animal variability and can ultimately provide the most clinically relevant data that cannot be obtained by any other means. The broad range of parameters that needs to be investigated in preclinical exams of these devices leave much room for innovation and advancement of this relatively young field. As implantable technologies mature and become ever more complex, new imaging strategies will continue to be critical to their advancement. In particular, imaging tools that can provide functional information about local implant performance and therapeutic efficacy combined are and will continue to be in great demand in drug delivery research and have the potential to speed up the transition into the clinical setting.
Fig. 5.
Summary of the various applications of biomedical imaging advancing research and clinical translation of implantable drug delivery systems.
Acknowledgments
This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award numbers R01EB016960, T32-EB007509-05 and by the Department of Defense Ovarian Cancer Research Program under award number DOD-WX81XWH-12-1-0500. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense or the National Institutes of Health.
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Weinberg BD, Blanco E, Gao J. Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci. 2008;97(5):1681–702. doi: 10.1002/jps.21038. [DOI] [PubMed] [Google Scholar]
- 2.Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pharm Sci. 2010;99(5):2219–39. doi: 10.1002/jps.21987. [DOI] [PubMed] [Google Scholar]
- 3.Abizaid A, Costa JR., Jr New drug-eluting stents: an overview on biodegradable and polymer-free next-generation stent systems. Circ Cardiovasc Interv. 2010;3(4):384–93. doi: 10.1161/CIRCINTERVENTIONS.109.891192. [DOI] [PubMed] [Google Scholar]
- 4.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25(6):1539–60. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 5.Leong K, Langer R. Polymeric controlled drug delivery. Adv Drug Deliv Rev. 1988;1(3):199–233. [Google Scholar]
- 6.Kleiner LW, Wright JC, Wang Y. Evolution of implantable and insertable drug delivery systems. J Control Release. 2014;181:1–10. doi: 10.1016/j.jconrel.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132(3):153–63. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190C:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivin I. International experience with NORPLANT and NORPLANT-2 contraceptives. Stud Fam Plann. 1988;19(2):81–94. [PubMed] [Google Scholar]
- 10.Sivin I, Campodonico I, Kiriwat O, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Hum Reprod. 1998;13(12):3371–8. doi: 10.1093/humrep/13.12.3371. [DOI] [PubMed] [Google Scholar]
- 11.Meirik O, Fraser IS, d’Arcangues C Women WHOCoICf. Implantable contraceptives for women. Hum Reprod Update. 2003;9(1):49–59. doi: 10.1093/humupd/dmg004. [DOI] [PubMed] [Google Scholar]
- 12.Berg WA, Hamper UM. Norplant implants: sonographic identification and localization for removal. AJR Am J Roentgenol. 1995;164(2):419–20. doi: 10.2214/ajr.164.2.7839981. [DOI] [PubMed] [Google Scholar]
- 13.Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Lupron Study Group. Fertil Steril. 1990;54(3):419–27. doi: 10.1016/s0015-0282(16)53755-8. [DOI] [PubMed] [Google Scholar]
- 14.Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25–31. doi: 10.1016/s0090-4295(02)02396-8. [DOI] [PubMed] [Google Scholar]
- 15.Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 16.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 17.DiMasi JA. The value of improving the productivity of the drug development process: faster times and better decisions. Pharmacoeconomics. 2002;20 (Suppl 3):1–10. doi: 10.2165/00019053-200220003-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature Reviews Drug Discovery. 2004;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti S, Schellens JH. The impact of FDA and EMEA guidelines on drug development in relation to Phase 0 trials. Br J Cancer. 2007;97(5):577–81. doi: 10.1038/sj.bjc.6603925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmus MN. Unique Aspects of Biomaterials in the Safety and Efficacy of Medical Implant. In: Helmus MN, editor. Biomaterials in the Design and Reliability of Medical Devices:Eurekah. 2002. pp. 1–73. [Google Scholar]
- 21.Appel AA, Anastasio MA, Larson JC, Brey EM. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34(28):6615–30. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deckers R, Moonen CT. Ultrasound triggered, image guided, local drug delivery. J Control Release. 2010;148(1):25–33. doi: 10.1016/j.jconrel.2010.07.117. [DOI] [PubMed] [Google Scholar]
- 23.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–60. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 24.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials. 2008;29(29):4012–21. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65(7):500–16. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Licha K, Olbrich C. Optical imaging in drug discovery and diagnostic applications. Adv Drug Deliv Rev. 2005;57(8):1087–108. doi: 10.1016/j.addr.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Burghardt AJ, Link TM, Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res. 2011;469(8):2179–93. doi: 10.1007/s11999-010-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RB, Solorio L, Wu H, Krupka T, Exner AA. Effect of injection site on in situ implant formation and drug release in vivo. J Control Release. 2010;147(3):350–8. doi: 10.1016/j.jconrel.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales-Rosello J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430–1. doi: 10.1016/j.contraception.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Eppel W, Sam C, Kratochwil A, Deutinger J, Bernaschek G. Intrauterine device localization by three-dimensional transvaginal sonography. Ultrasound Obstet Gynecol. 1997;10(4):289–92. doi: 10.1046/j.1469-0705.1997.10040289.x. [DOI] [PubMed] [Google Scholar]
- 31.Avitabile T, Marano F, Castiglione F, et al. Biocompatibility and biodegradation of intravitreal hyaluronan implants in rabbits. Biomaterials. 2001;22(3):195–200. doi: 10.1016/s0142-9612(00)00169-1. [DOI] [PubMed] [Google Scholar]
- 32.Solorio L, Babin BM, Patel RB, Mach J, Azar N, Exner AA. Noninvasive characterization of in situ forming implants using diagnostic ultrasound. J Control Release. 2010;143(2):183–90. doi: 10.1016/j.jconrel.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solorio L, Olear AM, Hamilton JI, et al. Noninvasive characterization of the effect of varying PLGA molecular weight blends on in situ forming implant behavior using ultrasound imaging. Theranostics. 2012;2(11):1064–77. doi: 10.7150/thno.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26(10):1389–401. doi: 10.7863/jum.2007.26.10.1389. [DOI] [PubMed] [Google Scholar]
- 35.Schiesser M, Lapaire O, Tercanli S, Holzgreve W. Lost intrauterine devices during pregnancy: maternal and fetal outcome after ultrasound-guided extraction. An analysis of 82 cases. Ultrasound Obstet Gynecol. 2004;23(5):486–9. doi: 10.1002/uog.1036. [DOI] [PubMed] [Google Scholar]
- 36.Bonilla-Musoles F, Raga F, Osborne NG, Blanes J. Control of intrauterine device insertion with three-dimensional ultrasound: is it the future? J Clin Ultrasound. 1996;24(5):263–7. doi: 10.1002/(SICI)1097-0096(199606)24:5<263::AID-JCU6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Stone NN, Stock RG. Brachytherapy for prostate cancer: real-time three-dimensional interactive seed implantation. Tech Urol. 1995;1(2):72–80. [PubMed] [Google Scholar]
- 38.Serruys PW, Onuma Y, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58(15):1578–88. doi: 10.1016/j.jacc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Honda Y, Grube E, de La Fuente LM, Yock PG, Stertzer SH, Fitzgerald PJ. Novel drug-delivery stent: intravascular ultrasound observations from the first human experience with the QP2-eluting polymer stent system. Circulation. 2001;104(4):380–3. doi: 10.1161/hc2901.094149. [DOI] [PubMed] [Google Scholar]
- 40.Oe K, Miwa M, Nagamune K, et al. Nondestructive evaluation of cell numbers in bone marrow stromal cell/beta-tricalcium phosphate composites using ultrasound. Tissue Eng Part C Methods. 2010;16(3):347–53. doi: 10.1089/ten.TEC.2008.0564. [DOI] [PubMed] [Google Scholar]
- 41.Kempe S, Metz H, Pereira PG, Mader K. Non-invasive in vivo evaluation of in situ forming PLGA implants by benchtop magnetic resonance imaging (BT-MRI) and EPR spectroscopy. Eur J Pharm Biopharm. 2010;74(1):102–8. doi: 10.1016/j.ejpb.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Solorio L, Olear AM, Zhou H, Beiswenger AC, Exner AA. Effect of cargo properties on in situ forming implant behavior determined by noninvasive ultrasound imaging. Drug Deliv Transl Res. 2012;2(1):45–55. doi: 10.1007/s13346-011-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Takanari K, Hong Y, et al. Non-invasive characterization of polyurethane-based tissue constructs in a rat abdominal repair model using high frequency ultrasound elasticity imaging. Biomaterials. 2013;34(11):2701–9. doi: 10.1016/j.biomaterials.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K, Jeong CG, Hollister SJ. Non-invasive monitoring of tissue scaffold degradation using ultrasound elasticity imaging. Acta Biomater. 2008;4(4):783–90. doi: 10.1016/j.actbio.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu LS, Kost J, Demanuele A, Langer R. Experimental Approach to Elucidate the Mechanism of Ultrasound-Enhanced Polymer Erosion and Release of Incorporated Substances. Macromolecules. 1992;25(1):123–8. [Google Scholar]
- 46.Kost J, Leong K, Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc Natl Acad Sci USA. 1989;86(20):7663–6. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyazaki S, Hou WM, Takada M. Controlled drug release by ultrasound irradiation. Chem Pharm Bull (Tokyo) 1985;33(1):428–31. doi: 10.1248/cpb.33.428. [DOI] [PubMed] [Google Scholar]
- 48.Lavon I, Kost J. Mass transport enhancement by ultrasound in non-degradable polymeric controlled release systems. J Control Release. 1998;54(1):1–7. doi: 10.1016/s0168-3659(97)00112-0. [DOI] [PubMed] [Google Scholar]
- 49.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–3. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 50.Acharya R, Wasserman R, Stevens J, Hinojosa C. Biomedical imaging modalities: a tutorial. Comput Med Imaging Graph. 1995;19(1):3–25. doi: 10.1016/0895-6111(94)00043-3. [DOI] [PubMed] [Google Scholar]
- 51.Richardson JC, Bowtell RW, Mader K, Melia CD. Pharmaceutical applications of magnetic resonance imaging (MRI) Adv Drug Deliv Rev. 2005;57(8):1191–209. doi: 10.1016/j.addr.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 52.du Toit LC, Carmichael T, Govender T, Kumar P, Choonara YE, Pillay V. In vitro, in vivo, and in silico evaluation of the bioresponsive behavior of an intelligent intraocular implant. Pharm Res. 2014;31(3):607–34. doi: 10.1007/s11095-013-1184-3. [DOI] [PubMed] [Google Scholar]
- 53.Chen SH, Lei M, Xie XH, et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013;9(5):6711–22. doi: 10.1016/j.actbio.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Hyde TM, Gladden LF, Payne R. A Nuclear-Magnetic-Resonance Imaging Study of the Effect of Incorporating a Macromolecular Drug in Poly(Glycolic Acid-Co-Dl-Lactic Acid) J Control Release. 1995;36(3):261–75. [Google Scholar]
- 55.Djemai A, Gladden LF, Booth J, Kittlety RS, Gellert PR. MRI investigation of hydration and heterogeneous degradation of aliphatic polyesters derived from lactic and glycolic acids: a controlled drug delivery device. Magn Reson Imaging. 2001;19(3–4):521–3. doi: 10.1016/s0730-725x(01)00283-1. [DOI] [PubMed] [Google Scholar]
- 56.Milroy GE, Cameron RE, Mantle MD, Gladden LF, Huatan H. The distribution of water in degrading polyglycolide. Part II: magnetic resonance imaging and drug release. J Mater Sci Mater Med. 2003;14(5):465–73. doi: 10.1023/a:1023223220409. [DOI] [PubMed] [Google Scholar]
- 57.Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187–92. doi: 10.1038/nature11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Robinson MR, Lizak MJ, et al. Controlled drug release from an ocular implant: an evaluation using dynamic three-dimensional magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2004;45(8):2722–31. doi: 10.1167/iovs.04-0091. [DOI] [PubMed] [Google Scholar]
- 59.Kim H, Lizak MJ, Tansey G, et al. Study of ocular transport of drugs released from an intravitreal implant using magnetic resonance imaging. Ann Biomed Eng. 2005;33(2):150–64. doi: 10.1007/s10439-005-8974-7. [DOI] [PubMed] [Google Scholar]
- 60.Reisfeld B, Blackband S, Calhoun V, Grossman S, Eller S, Leong K. The use of magnetic resonance imaging to track controlled drug release and transport in the brain. Magn Reson Imaging. 1993;11(2):247–52. doi: 10.1016/0730-725x(93)90029-d. [DOI] [PubMed] [Google Scholar]
- 61.Giers MB, McLaren AC, Schmidt KJ, Caplan MR, McLemore R. Distribution of molecules locally delivered from bone cement. J Biomed Mater Res B Appl Biomater. 2014;102(4):806–14. doi: 10.1002/jbm.b.33062. [DOI] [PubMed] [Google Scholar]
- 62.van der Zande M, Sitharaman B, Walboomers XF, et al. In vivo Magnetic Resonance Imaging of the Distribution Pattern of Gadonanotubes Released from a Degrading Poly(Lactic-Co-Glycolic Acid) Scaffold. Tissue Eng Part C Methods. 2011;17(1):19–26. doi: 10.1089/ten.TEC.2010.0089. [DOI] [PubMed] [Google Scholar]
- 63.Giers MB, Estes CS, McLaren AC, Caplan MR, McLemore R. Jeannette Wilkins Award: Can locally delivered gadolinium be visualized on MRI? A pilot study. Clin Orthop Relat Res. 2012;470(10):2654–62. doi: 10.1007/s11999-012-2315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weissleder R, Poss K, Wilkinson R, Zhou C, Bogdanov A., Jr Quantitation of slow drug release from an implantable and degradable gentamicin conjugate by in vivo magnetic resonance imaging. Antimicrob Agents Chemother. 1995;39(4):839–45. doi: 10.1128/aac.39.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giers MB, Estes CS, McLaren AC, Caplan MR, McLemore R. Jeannette Wilkins Award: Can Locally Delivered Gadolinium Be Visualized on MRI? A Pilot Study. Clin Orthop Relat Res. 2012;470(10):2654–62. doi: 10.1007/s11999-012-2315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lalande C, Miraux S, Derkaoui SM, et al. Magnetic resonance imaging tracking of human adipose derived stromal cells within three-dimensional scaffolds for bone tissue engineering. Eur Cell Mater. 2011;21:341–54. doi: 10.22203/ecm.v021a25. [DOI] [PubMed] [Google Scholar]
- 67.Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5(6):567–84. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 68.Hoehn M, Kustermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99(25):16267–72. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langer R, Hsieh DST, Rhine W, Folkman J. Control of release kinetics of macromolecules from polymers. J Memb Sci. 1980;7(3):333–50. [Google Scholar]
- 70.Edelman ER, Langer R. Optimization of release from magnetically controlled polymeric drug release devices. Biomaterials. 1993;14(8):621–6. doi: 10.1016/0142-9612(93)90182-2. [DOI] [PubMed] [Google Scholar]
- 71.Kost J, Wolfrum J, Langer R. Magnetically enhanced insulin release in diabetic rats. J Biomed Mater Res. 1987;21(12):1367–73. doi: 10.1002/jbm.820211202. [DOI] [PubMed] [Google Scholar]
- 72.Saslawski O, Weingarten C, Benoit JP, Couvreur P. Magnetically Responsive Microspheres for the Pulsed Delivery of Insulin. Life Sciences. 1988;42(16):1521–8. doi: 10.1016/0024-3205(88)90009-4. [DOI] [PubMed] [Google Scholar]
- 73.Qian F, Stowe N, Liu EH, Saidel GM, Gao J. Quantification of in vivo doxorubicin transport from PLGA millirods in thermoablated rat livers. J Control Release. 2003;91(1–2):157–66. doi: 10.1016/s0168-3659(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 74.Gao J, Qian F, Szymanski-Exner A, Stowe N, Haaga J. In vivo drug distribution dynamics in thermoablated and normal rabbit livers from biodegradable polymers. J Biomed Mater Res. 2002;62(2):308–14. doi: 10.1002/jbm.10292. [DOI] [PubMed] [Google Scholar]
- 75.Weinberg BD, Blanco E, Lempka SF, Anderson JM, Exner AA, Gao J. Combined radiofrequency ablation and doxorubicin-eluting polymer implants for liver cancer treatment. J Biomed Mater Res A. 2007;81(1):205–13. doi: 10.1002/jbm.a.30926. [DOI] [PubMed] [Google Scholar]
- 76.Artzi N, Oliva N, Puron C, et al. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat Mater. 2011;10(9):704–9. doi: 10.1038/nmat3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eisenacher F, Schadlich A, Mader K. Monitoring of internal pH gradients within multi-layer tablets by optical methods and EPR imaging. Int J Pharm. 2011;417(1–2):204–15. doi: 10.1016/j.ijpharm.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Schadlich A, Kempe S, Mader K. Non-invasive in vivo characterization of microclimate pH inside in situ forming PLGA implants using multispectral fluorescence imaging. J Control Release. 2014;179:52–62. doi: 10.1016/j.jconrel.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 79.Sjollema J, Sharma PK, Dijkstra RJ, et al. The potential for bio-optical imaging of biomaterial-associated infection in vivo. Biomaterials. 2010;31(8):1984–95. doi: 10.1016/j.biomaterials.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Zhang Y, Gautam S, et al. Development of aliphatic biodegradable photoluminescent polymers. Proc Natl Acad Sci USA. 2009;106(25):10086–91. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okamura T, Onuma Y, Garcia-Garcia HM, et al. 3-Dimensional optical coherence tomography assessment of jailed side branches by bioresorbable vascular scaffolds: a proposal for classification. JACC Cardiovasc Interv. 2010;3(8):836–44. doi: 10.1016/j.jcin.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Okamura T, Serruys PW, Regar E. Cardiovascular flashlight. The fate of bioresorbable struts located at a side branch ostium: serial three-dimensional optical coherence tomography assessment. Eur Heart J. 2010;31(17):2179. doi: 10.1093/eurheartj/ehq175. [DOI] [PubMed] [Google Scholar]
- 83.Barlis P, Regar E, Serruys PW, et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31(2):165–76. doi: 10.1093/eurheartj/ehp480. [DOI] [PubMed] [Google Scholar]
- 84.Guagliumi G, Ikejima H, Sirbu V, et al. Impact of drug release kinetics on vascular response to different zotarolimus-eluting stents implanted in patients with long coronary stenoses: the LongOCT study (Optical Coherence Tomography in Long Lesions) JACC Cardiovasc Interv. 2011;4(7):778–85. doi: 10.1016/j.jcin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Patterson J, Stayton PS, Li X. In situ characterization of the degradation of PLGA microspheres in hyaluronic acid hydrogels by optical coherence tomography. IEEE Trans Med Imaging. 2009;28(1):74–81. doi: 10.1109/TMI.2008.927356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen CW, Betz MW, Fisher JP, Paek A, Chen Y. Macroporous Hydrogel Scaffolds and Their Characterization By Optical Coherence Tomography. Tissue Eng Part C Methods. 2011;17(1):101–12. doi: 10.1089/ten.TEC.2010.0072. [DOI] [PubMed] [Google Scholar]
- 87.Ghosn MG, Tuchin VV, Larin KV. Nondestructive quantification of analyte diffusion in cornea and sclera using optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48(6):2726–33. doi: 10.1167/iovs.06-1331. [DOI] [PubMed] [Google Scholar]
- 88.Beeley NR, Stewart JM, Tano R, et al. Development, implantation, in vivo elution, and retrieval of a biocompatible, sustained release subretinal drug delivery system. J Biomed Mater Res A. 2006;76(4):690–8. doi: 10.1002/jbm.a.30567. [DOI] [PubMed] [Google Scholar]
- 89.Shanti NO, Chan VWL, Stock SR, De Carlo F, Thornton K, Faber KT. X-ray micro-computed tomography and tortuosity calculations of percolating pore networks. Acta Materialia. 2014;71(0):126–35. [Google Scholar]
- 90.Wang Y, Wertheim DF, Jones AS, Coombes AG. Micro-CT in drug delivery. Eur J Pharm Biopharm. 2010;74(1):41–9. doi: 10.1016/j.ejpb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Chang HI, Wertheim DF, Jones AS, Jackson C, Coombes AG. Characterisation of the macroporosity of polycaprolactone-based biocomposites and release kinetics for drug delivery. Biomaterials. 2007;28(31):4619–27. doi: 10.1016/j.biomaterials.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 92.Haesslein A, Ueda H, Hacker MC, et al. Long-term release of fluocinolone acetonide using biodegradable fumarate-based polymers. J Control Release. 2006;114(2):251–60. doi: 10.1016/j.jconrel.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 93.Krebs MD, Sutter KA, Lin AS, Guldberg RE, Alsberg E. Injectable poly(lactic-co-glycolic) acid scaffolds with in situ pore formation for tissue engineering. Acta Biomater. 2009;5(8):2847–59. doi: 10.1016/j.actbio.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Wertheim DF, Jones AS, Chang HI, Coombes AG. Micro-CT analysis of matrix-type drug delivery devices and correlation with protein release behaviour. J Pharm Sci. 2010;99(6):2854–62. doi: 10.1002/jps.22027. [DOI] [PubMed] [Google Scholar]
- 95.Vallejo-Heligon SG, Klitzman B, Reichert WM. Characterization of porous, dexamethasone-releasing polyurethane coatings for glucose sensors. Acta Biomater. 2014;10(11):4629–38. doi: 10.1016/j.actbio.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holloway JL, Ma H, Rai R, Burdick JA. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J Control Release. 2014;191:63–70. doi: 10.1016/j.jconrel.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wada K, Yu W, Elazizi M, et al. Locally delivered salicylic acid from a poly(anhydride-ester): impact on diabetic bone regeneration. J Control Release. 2013;171(1):33–7. doi: 10.1016/j.jconrel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gauthier O, Muller R, von Stechow D, et al. In vivo bone regeneration with injectable calcium phosphate biomaterial: a three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26(27):5444–53. doi: 10.1016/j.biomaterials.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 99.Peter B, Gauthier O, Laib S, et al. Local delivery of bisphosphonate from coated orthopedic implants increases implants mechanical stability in osteoporotic rats. J Biomed Mater Res A. 2006;76(1):133–43. doi: 10.1002/jbm.a.30456. [DOI] [PubMed] [Google Scholar]
- 100.Lee YH, Bhattarai G, Park IS, et al. Bone regeneration around N-acetyl cysteine-loaded nanotube titanium dental implant in rat mandible. Biomaterials. 2013;34(38):10199–208. doi: 10.1016/j.biomaterials.2013.08.080. [DOI] [PubMed] [Google Scholar]
- 101.Exner AA, Weinberg BD, Stowe NT, et al. Quantitative computed tomography analysis of local chemotherapy in liver tissue after radiofrequency ablation. Acad Radiol. 2004;11(12):1326–36. doi: 10.1016/j.acra.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 102.Szymanski-Exner A, Stowe NT, Lazebnik RS, et al. Noninvasive monitoring of local drug release in a rabbit radiofrequency (RF) ablation model using X-ray computed tomography. J Control Release. 2002;83(3):415–25. doi: 10.1016/s0168-3659(02)00216-x. [DOI] [PubMed] [Google Scholar]
- 103.Szymanski-Exner A, Stowe NT, Salem K, et al. Noninvasive monitoring of local drug release using X-ray computed tomography: optimization and in vitro/in vivo validation. J Pharm Sci. 2003;92(2):289–96. doi: 10.1002/jps.10295. [DOI] [PubMed] [Google Scholar]
- 104.Nakamura H, Hashimoto T, Oi H, Sawada S. Iodized oil in the portal vein after arterial embolization. Radiology. 1988;167(2):415–7. doi: 10.1148/radiology.167.2.2833765. [DOI] [PubMed] [Google Scholar]
- 105.Jeon UB, Lee JW, Choo KS, et al. Iodized oil uptake assessment with cone-beam CT in chemoembolization of small hepatocellular carcinomas. World J Gastroenterol. 2009;15(46):5833–7. doi: 10.3748/wjg.15.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim HK, Han JK. Hepatocellular carcinoma: evaluation of therapeutic response to interventional procedures. Abdom Imaging. 2002;27(2):168–79. doi: 10.1007/s00261-001-0093-9. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Bella E, Lee CS, et al. The synergistic effects of 3-D porous silk fibroin matrix scaffold properties and hydrodynamic environment in cartilage tissue regeneration. Biomaterials. 2010;31(17):4672–81. doi: 10.1016/j.biomaterials.2010.02.006. [DOI] [PubMed] [Google Scholar]