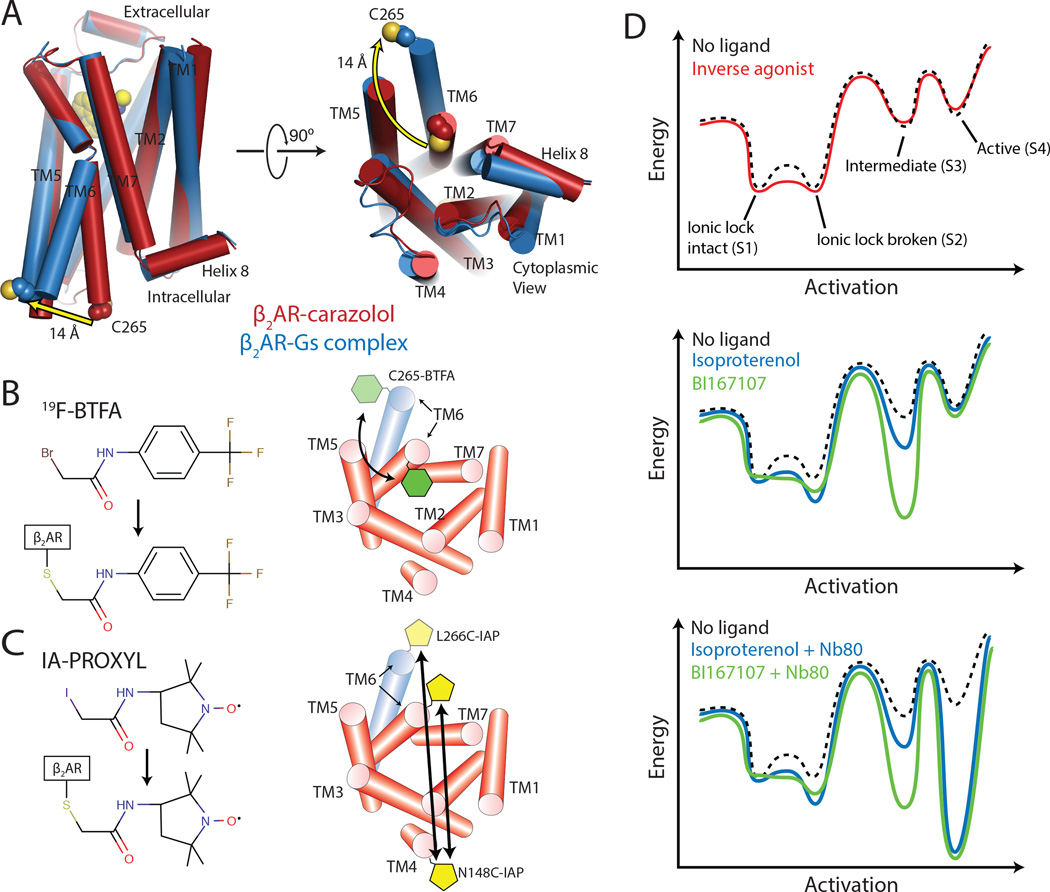
Figure 1.
Spectroscopic methods for detecting conformational changes of β2AR.
(A) Comparison of crystal structures of inactive, carazolol-bound and active β2AR in complex with agonist BI167107 and Gs. The crystal structures reveal a 14 Å outward displacement of TM6 upon β2AR activation. Cys265, used for 19F-NMR experiments is highlighted in spheres.
(B) 19F-NMR studies utilize the fluorine label 2-bromo-4-(trifluoromethyl)acetanilide (19F-BTFA) that reports changes in the chemical environment at the cytoplasmic end of TM6. See Figure S1 and Table S1 for construct design and validation.
(C) For DEER spectroscopy, β2AR was labeled at the cytoplasmic ends of TM4 (site N148C-IAP) and TM6 (site L266C-IAP) with the nitroxide label 3-(2-iodoacetamido)-2,2,5,5-tetramethylpyrroline-1-oxyl (IA-PROXYL).
(D) Energy landscape of β2AR in the presence of inverse agonists carazolol and ICI-118,551, agonists isoproterenol and BI167107, and agonists with Nb80.