Abstract
Purpose
Diagnosis of Rett syndrome (RTT) is often delayed. We sought to determine type of physician who typically makes the diagnosis of RTT and to identify risk factors for delayed diagnosis.
Methods
One-thousand eighty-five participants from the multicenter longitudinal RTT natural history study with classic and atypical RTT were recruited from 2006 to 2014. Age of diagnosis, diagnostician, diagnostic criteria, clinical and developmental data were collected.
Results
Among 919 classic and 166 atypical RTT participants, median diagnosis age was 2.7 years (interquartile range 2.0–4.1) in classic and 3.8 years (interquartile range 2.3–6.9) in atypical RTT. Pediatricians made the diagnosis of classic RTT rarely (5.2%); however, proportion diagnosed by pediatricians increased since 2006. Since the first diagnostic criteria, the age of diagnosis decreased among subspecialists but not pediatricians. Odds of a pediatrician making the diagnosis of classic RTT were higher if a child stopped responding to parental interaction, and lower if they possessed gastro-esophageal reflux, specific stereotypies, lost babbling or the ability to follow commands. Delayed acquisition of basic gross motor skills or finger feeding were associated with younger diagnosis; delayed acquisition of higher level fine motor skills, later onset of supportive features, and normal head circumference were associated with late diagnosis. 33% with microcephaly before 2.5 years were diagnosed after the median age of 2.7 years.
Conclusions
Age of RTT diagnosis has improved among subspecialists, and pediatricians have made the diagnosis of classic RTT more frequently since 2006. Strategies for educating diagnosticians should incorporate specific risk factors for delayed diagnosis.
Keywords: Rett syndrome, MECP2, early diagnosis, risk factors, prognosis
Introduction and Objectives
Rett syndrome (RTT), the leading cause of profound intellectual disability in females, is characterized by apparently normal early development followed by psychomotor regression. Despite association with mutations in the methyl-CpG-binding protein 2 gene (MECP2) in the majority, the diagnosis of RTT remains clinical.1 Regression and midline hand stereotypies typically commence between 12–24 months, but can begin after 4 years.2 Moreover, nonspecific developmental abnormalities can be present prior to 6 months.3 Mutation type, associated with age of onset of regression and hand stereotypies, accounts for some variability in age of presentation.4,5
Reports on average age of diagnosis are limited; age of diagnosis in Australia decreased from a mean of 10.1 years for those born before 1980 to 2.5 years for those born between 2004 and 2006, possibly due to updates to RTT diagnostic criteria and the introduction of genetic testing.6 Rett syndrome presents with a broad range of features, and 2–4 years may pass between initial presentation and diagnosis.7 In Australia, delayed diagnosis has been associated with year of birth,6 late or atypical presenting features,7 and onset of developmental milestones and stereotypies.8 However, no US study has examined risk factors for delayed diagnosis, whether pediatricians or subspecialists typically make the diagnosis, or the impact of developmental screening strategies on age of RTT diagnosis.
Early identification of developmental disorders is an important role of pediatricians;9 they are the gatekeepers to further access to services. Data on age of diagnosis and factors associated with delayed diagnosis could raise awareness about the presentation of RTT. The American Academy of Pediatrics (AAP) has recommended developmental surveillance and screening beginning at 9 months of age,9 which raises two questions. Are children with RTT, who typically present with both developmental delay and regression, being detected by pediatricians? If so, do specific characteristics guide pediatricians to diagnose?
To improve appreciation of the clinical presentation of RTT and recognition of specific features among US healthcare providers, we examined age of diagnosis and associated factors in a large US cohort. The aims of this study were two-fold: 1) to investigate the influence of clinical, demographic and socioeconomic features, as well as changes in diagnostic criteria on age of diagnosis, and 2) to determine what type of physician made the initial diagnosis. We hypothesized that specific clinical features and patterns of development are associated with age of diagnosis and what type of physician makes the diagnosis. We also explored the influence of genetic testing and revision of developmental screening strategies on age of diagnosis.
Methods
Study Design and Participants
Participants were recruited, as described previously,10 from 2006 to 2014 through the multicenter RTT natural history study (RNHS) at one of eight US sites and evaluated every six months until age 6 and every twelve months thereafter. All participants had MECP2 testing. An RNHS neurologist or geneticist characterized diagnosis based on consensus criteria.1,11 Participants with clinical classic or atypical RTT were analyzed, regardless of MECP2 results, but those with other mutations were excluded; summary data were collected for males, those with MECP2 duplication, and those with MECP2 mutation who did not fulfill clinical criteria for RTT (non-RTT).
The age of RTT diagnosis and developmental history were obtained using a combination of family or caregiver reports, baby books, photos or videos, MECP2 testing dates, and clinician notes. If age of diagnosis was not available, a surrogate was based on MECP2 testing date, and the requesting physician was credited with the diagnosis. Demographic data included race and ethnicity, type of residence, and parental age. Median income and population density were estimated using address. At each visit, a RNHS physician completed neurological exam, an anthropometrist recorded somatic measurements, and two quantitative scales of disease severity, the motor behavioral assessment and clinical severity scale described previously,10 were administered. Each institutional review board approved the study, and the RNHS clinician verified all data.
Data Categorization
The period of diagnosis was categorized based on historical events (i.e., secular variation, Table 1).1,9,11–17 Normative18 and RTT-specific10 growth Z-scores were calculated. Developmental acquisitions were categorized based on Denver-II percentile19 as normal (<75th), concerning (75th to 90th) or delayed (>90th).
Table 1.
Historical Period and Age of Diagnosis by Subspecialists
| Period | N | Median Age of Diagnosis (y) |
Mean Rank |
Significantly different from periods: |
|---|---|---|---|---|
| A: 1983a – 1984 | 13 | 6.33 | 645.8 | F*, G**, H**, I** |
| B: 1985b – 1987 | 50 | 6.17 | 591.0 | D**, F**, G***, H***, I*** |
| C: 1988c – 1994 | 84 | 3.08 | 494.6 | G**, H***, I** |
| D: 1995d – 1998 | 64 | 2.91 | 410.3 | B** |
| E: 1999e – 2000 | 88 | 3.08 | 471.3 | H** |
| F: 2001f | 39 | 2.50 | 376.7 | A*, B** |
| G: 2002g – 2005 | 196 | 2.50 | 377.1 | A**, B***, C** |
| H: 2006h – 2009 | 194 | 2.41 | 355.9 | A**, B***, C***, E** |
| I: 2010i – 2014 | 96 | 2.54 | 364.8 | A**, B***, C** |
| Total | 824 | 2.75 | ||
Periods divided based on:
1983, first description of the disorder in English;
1985, first diagnostic criteria for diagnosis;
1988, update to diagnostic criteria;
1995, atypical RTT criteria;
1999, association between MECP2 mutations and RTT;
2001, clinical availability of MECP2 testing and AAP developmental screening recommendations;
2002, update to diagnostic criteria;
2006, AAP routine screening algorithm for developmental disorders;
2010, update to diagnostic criteria.
p - value <.05,
p - value <.01,
p - value <.001
Statistical Analysis
Descriptive analyses were performed. Age of diagnosis distribution is positively skewed, so nonparametric analyses were performed when possible. Kruskal-Wallis H was used to evaluate the association between categories (e.g., diagnosis, period effect) and age of diagnosis, and Mann-Whitney U tests (with Bonferroni correction) were used for post-hoc and other comparisons between two groups. Logistic regression was used to determine which Rett-related features and developmental milestones predict whether the diagnosis of classic RTT was made by a pediatrician or specialist. Nonparametric correlation (Kendall's τb) was used to compare continuous variables such as age of diagnosis and age of onset of RTT characteristics (with Bonferroni correction). Predictors were included in regression models if p-value was <.10, and p-value of < .05 was considered statistically significant for all other comparisons. Nonparametric comparisons are summarized using median and interquartile range (IQR). Analyses were performed using SPSS version 21,20 ArcMap Editor,21 and Address Coder Premium.22
Results
Among 1205 participants, 21 were excluded due to incomplete data, and 2 with CDKL5 mutation and atypical RTT were excluded. The single male with atypical RTT, 61 Non-RTT and 35 duplication participants were excluded from analysis, but age of diagnosis is summarized in eTable 1 (supplementary material). Median age of diagnosis was 5.4 years for Non-RTT females, 3.5 years for Non-RTT males, 37.8 years for duplication females, and 7.3 years for duplication males. Remaining female participants (919 classic RTT and 166 atypical RTT) were followed for up to 8.2 years (median 4.0y). Birth year ranged from 1943 to 2012 (median 2001), and participants were between 8 months and 66.5 years old at enrollment (median 6.8y). Demographics are summarized in eTable 2, and participants were mostly Caucasian, non-Hispanic (supplementary material).
Characteristics of diagnosis
Distribution
Participants were diagnosed between 1983 and 2013. Age of diagnosis ranged from 7 months to 53.0 years. Median age of diagnosis was 2.7 years (IQR 2.0 – 4.1) in classic and 3.8 years (IQR 2.3 – 6.9) in atypical RTT (Table 2).
Table 2.
Diagnostician and age of diagnosis.
| Classic RTT | |||||||
| Diagnostician | Number | Percent |
Median Age (y) |
Interquartile Range |
Minimum Age |
Maximum Age |
Mean Rank* |
| Pediatrician | 48 | 5.2% | 2.4 | 1.8–3.5 | 1.2 | 18.1 | 379.4 |
| Developmental pediatrician | 273 | 29.7% | 3.0 | 2.0–4.2 | 0.6 | 34.1 | 465.0 |
| Neurologist | 324 | 35.3% | 2.7 | 2.0–4.4 | 0.8 | 31.0 | 446.0 |
| Geneticist | 204 | 22.2% | 2.5 | 2.0–3.5 | 0.9 | 40.0 | 415.6 |
| Other specialist | 23 | 2.5% | 2.8 | 2.1–4.5 | 1.5 | 14.8 | 465.8 |
| Other primary care provider | 2 | 0.2% | n.c. | n.c. | 3.1 | 20.0 | 703.8 |
| Family member or teacher | 12 | 1.3% | 3.3 | 2.0–13.7 | 1.3 | 53.0 | 530.3 |
| Missing | 16 | 1.7% | |||||
| Overall | 919 | 100.0% | 2.7 | 2.0–4.1 | 0.6 | 53.0 | |
| Atypical RTT | |||||||
| Diagnostician | Number | Percent |
Median Age (y) |
Interquartile Range |
Minimum Age |
Maximum Age |
Mean Rank* |
| Pediatrician | 4 | 2.4% | 5.7 | 1.6–35.4 | 1.5 | 44.1 | 84.0 |
| Developmental pediatrician | 50 | 30.1% | 5.1 | 2.5–8.1 | 1.4 | 37.1 | 90.1 |
| Neurologist | 55 | 33.1% | 3.5 | 2.4–6.1 | 1.0 | 30.8 | 76.5 |
| Geneticist | 44 | 26.5% | 2.9 | 2.1–5.1 | 0.7 | 27.0 | 67.2 |
| Other specialist | 3 | 1.8% | 7.0 | n.c. | 5.3 | 10.0 | 120.8 |
| Other primary care provider | 1 | 0.6% | 25.8 | n.c. | n.c. | n.c. | 154.0 |
| Family member or teacher | 1 | 0.6% | 8.0 | n.c. | n.c. | n.c. | 128.0 |
| Missing | 8 | 4.8% | |||||
| Overall | 166 | 100.0% | 3.8 | 2.3–6.9 | 0.7 | 44.0 | |
p = .06, n.c. = not calculated
Who made the diagnosis
Diagnosis was typically made by a neurologist, developmental pediatrician, or geneticist, and infrequently by a primary care provider (Table 2). Odds of a pediatrician diagnosing classic RTT were lower than specialists if the child had lost babbling or the ability to follow commands with a gesture, or if gastro-esophageal reflux (GER) was present (Table 3). Additionally, pediatricians were less likely to diagnose classic RTT if clasping, posturing, clapping or tapping stereotypies were present before diagnosis, but equally likely to diagnose if common stereotypies (hand wringing) were present. Odds of a pediatrician making the diagnosis were higher if the child had lost the ability to be consoled by being held, or stopped reacting to the parents’ voice or the command “no”. Insufficient atypical participants existed for logistic regression. The age of diagnosis was similar among all diagnosticians for both classic (χ2(6) = 11.02, p = .09) and atypical RTT (χ2(6) = 12.28, p = .06).
Table 3.
Odds of a pediatrician making the diagnosis of classic RTT based on specific characteristics
| Characteristic | Odds Ratio | p-value | 95% CI | |
|---|---|---|---|---|
| Regression | Loss of ability to quiet to parent's voice | 2.5 | .009 | 1.3–5.0 |
| Loss of ability to inhibit to "no" | 3.8 | .001 | 1.8–8.1 | |
| Loss of affinity for being held | 3.1 | .001 | 1.6–5.9 | |
| Loss of babbling | 0.6 | .09 | 0.3–1.1 | |
| Loss of ability to follow a command with a gesture | 0.4 | .04 | 0.1–0.9 | |
| Hand Stereotypies | Clapping or Tapping | 0.3 | .01 | 0.1–0.8 |
| Clasping or Posturing | 0.2 | .07 | 0.1–1.3 | |
| Supportive Features | Gastro-esophageal Reflux | 0.6 | .06 | 0.3–1.0 |
Non-significant predictor variables from regression are not shown.
Secular period
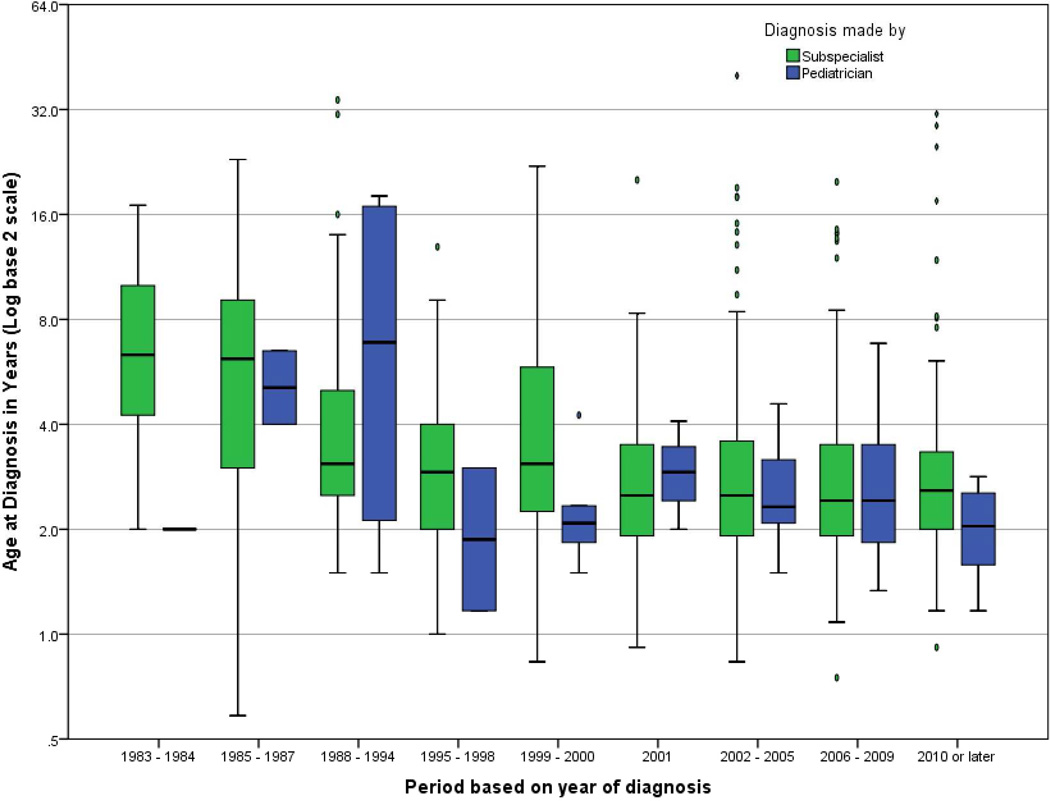
Proportion of pediatricians making the diagnosis of classic RTT was similar in all time periods prior to 2006 (4.1%), but increased after 2006 (8.2%, p = .02). Pediatricians diagnosed atypical RTT in 2.4% of cases, which did not change with time period. Median age of diagnosis of classic RTT by subspecialists varied with time period (χ2(8) = 76.10, p < .001, Figure); age of diagnosis declined after 1987, with stabilization after 2000, and no significant change in age of diagnosis from 2001 to the present (Table 1). Median age of diagnosis did not change for classic RTT diagnosed by a pediatrician, or for atypical RTT regardless of diagnostician (data not shown).
Figure. Age of classic RTT diagnosis and diagnostician, based on historical period.
FPO – VECTOR FILE IMAGES UPLOADED IN COLOR (FOR ONLINE-ONLY) AND B&W (FOR PRINT) VERSIONS
Ages of diagnosis differed for subspecialists based on period, demonstrating a decline in age of diagnosis with stabilization after 2000. No significant trend was present for pediatricians. Post-hoc comparisons are detailed in Table 1. Box-plots indicate median age and inter-quartile range, and whiskers extend 1.5× the inter-quartile range. Ovals indicate outliers, and diamonds indicate extreme outliers. (To be reproduced in color on the Web and in black-and-white in print)
Clinical characteristics
Age at diagnosis of classic RTT was younger in children with delayed acquisition of pulling to stand, supported walking, independent walking, or finger feeding, but older in children with delayed acquisition of pincer grasp or transfer of objects from hand to hand (Table 4). Age of onset of the following characteristics was correlated with age of diagnosis: hyperventilation (rτ [380] = .21), breath holding (rτ [470] = .25), air swallowing (rτ [318] = .25), drooling (rτ [528] = .20), bruxism (rτ [694] = .29), constipation (rτ [580] = .25), GER (rτ [432] = .26), bone fractures (rτ [136] = .27), stereotypies (rτ [851] = .30), self-abuse (rτ [428] = .24), scoliosis (rτ [300] = .23), developmental regression (rτ [874] = .21), and head circumference deceleration (rτ [668] = .11, all p < .001). Neither child’s nor parents’ quality of life was associated with age at diagnosis (data not shown).
Table 4.
Age of diagnosis of classic RTT among all diagnosticians in the presence of normal, concerning, or delayed acquisition of developmental milestones.
| Median Age of Diagnosis and Developmental Milestones | ||||
|---|---|---|---|---|
| Developmental milestone | Normal (IQR, n) | Concerning (IQR, n) | Delayed (IQR, n) | p-value |
| Pulling to stand | 3.6 (2.5–6.9, 109) | 2.8 (2.1–4.3, 311) | 2.6 (2.0–4.0, 89) | .01 |
| Walking with Support | 3.8 (2.3–6.5, 152) | 2.8 (2.1–4.0, 355) | 2.5 (2.0–4.0, 117) | <.001 |
| Independent walking | 9.0 (3.1–9.0, 3) | 3.5 (2.4–5.4, 309) | 2.9 (2.2–4.1, 160) | .04 |
| Finger feeding | 3.6 (2.5–6.9, 109) | 2.8 (2.1–4.3, 311) | 2.6 (2.0–4.0, 89) | .02 |
| Transfer from hand to hand | 2.5 (1.9–3.5, 387) | 2.1 (1.5–2.8, 26) | 3.3 (2.6–4.8, 42) | .002 |
| Pincer grasp | 2.5 (2.0–3.5, 306) | 2.7 (2.1–4.1, 132) | 3.6 (2.2–6.0, 56) | .008 |
Median age in years (IQR, n). p-value adjusted for multiple comparisons using Bonferroni correction.
Growth
Rett-specific height z-score (rτ [296] = .12, p = .04) and head circumference z-score (rτ [287] = .19, p = .001) at time of diagnosis were higher in those diagnosed at an older age. However, of the 16% (39/247) who exhibited acquired microcephaly (below 2nd percentile) before 2.5 years, 33.3% (13/39) were not diagnosed until after the median age of 2.7 years, and 46% (6/13) of these were not diagnosed until after the upper quartile of 4.1 years. Of the 83% (682/824) who eventually exhibited microcephaly, 19% (128/682) were not diagnosed until after 4.1 years.
Developmental delay, supportive features, and diagnosis of classic RTT
Because age of milestone and supportive feature acquisition were not recorded on all participants, hypothesis testing could not be performed; however, the average time from appearance of a characteristic to diagnosis is instructive. Most participants exhibited stereotypies and language regression prior to diagnosis (Table 5); stereotypies had been occurring for a median of 1.1 years (IQR 0.5–2.5y, n=685). The longest time from regression to diagnosis was for advanced skills: a median of 6.3 years after losing the ability to pedal a tricycle (IQR 1.7–9.6y, n=12), 1.7 years after losing phrases (IQR 0.6–4.7y, n=125), and 1.6 years after losing independent walking (IQR 0.4–3.8y, n=103). Time to diagnosis was shortest after loss of more fundamental skills: a median of 0.8 years after loss of finger feeding (IQR 0.3–1.6y, n=334), holding a bottle (IQR 0.3–2.0y, n=269), transferring objects from hand to hand (IQR 0.3–1.6y, n=287), and pulling to stand (IQR 0.4–2.6y, n=112), and a median of 0.9 years after loss of pincer grasp (IQR 0.3–2.0y, n=340) and reaching (IQR 0.4–2.1y, n=328). The longest median intervals after appearance of supportive features were for gallbladder dysfunction (3.3y, IQR 1.7–10.0y, n=5), scoliosis (1.8y, IQR 0.4–4.6y, n=76), self-abusive behaviors (1.8y, IQR 0.6–3.8y, n=55), GER (1.7y, IQR 1.0–2.9y, n=257), and bone fracture (1.6y, IQR 1.0–4.5, n=54), and the shortest median time was after appearance of finger-rubbing stereotypies (0.7y, IQR 0.3–2.0y, n=111), hyperventilation (0.9y, IQR 0.3–2.9y, n=207), breath-holding (1.0y, IQR 0.3–2.2y, n=259), or bruxism (1.0y, IQR 0.5–2.1y, n=478).
Table 5.
Characteristics present by history prior to diagnosis with classic RTT (n = 869*).
| Characteristic | Specific regression | Percent | |
|---|---|---|---|
| Core features | Stereotypies | 86.4% | |
| Fine motor regression | Overall | 77.0% | |
| Holding bottle | 32.2% | ||
| Pincer grasp | 40.8% | ||
| Finger feeding | 40.1% | ||
| Language regression | Overall | 87.3% | |
| Babbling | 37.9% | ||
| Single word with meaning | 58.7% | ||
| Phrases | 14.5% | ||
| Follow command with gesture | 16.2% | ||
| Follow command without gesture | 10.5% | ||
| Other regression | Gross motor regression** | Overall | 55.7% |
| Pull to sit | 9.0% | ||
| Crawling | 16.6% | ||
| Walk independently | 4.8% | ||
| Loss of attention | Visual | 22.8% | |
| Auditory | 19.8% | ||
| Supportive features | Bruxism | 56.7% | |
| Constipation | 43.4% | ||
| Self-abusive | 41.5% | ||
| Drooling | 39.2% | ||
| Breath-holding | 30.7% | ||
| Gastro-esophageal reflux | 30.6% | ||
| Hyperventilation | 24.6% | ||
| Aerophagia | 22.2% | ||
| Scoliosis | 8.9% | ||
| Bone fractures | 6.3% | ||
| Gallbladder dysfunction | 0.6% | ||
50 participants had incomplete data on development and supportive features
Gait apraxia (a core diagnostic feature) could not be evaluated retrospectively, therefore gross motor regression is reported.
Demographic and socioeconomic factors
Diagnosis was made at a younger age in classic RTT if either mother (rτ [704] = -.117, p < .001) or father (rτ [688] = −.104, p < .001) was older at participant’s birth, and at a younger age in atypical RTT if mother was older (rτ [111] = −.141, p = .024). Participants with an estimated household income above the national median were diagnosed earlier (median 2.5y, IQR 2.0–4.0y, n=522) than those with lower income (median 3.0y, IQR 2.1–4.6y, n=331, U = 76,748, p = .006). No influences of race, ethnicity, or population density on age of diagnosis were found.
Discussion
Early diagnosis in RTT offers many benefits. In addition to the opportunity for specific counseling about prognosis and potential comorbidities, many therapeutic strategies have proven effective in RTT, including physical, occupational,23 behavioral,24 and music therapy.25 Guidelines for tailored programs exist26, and can maximize therapeutic effect.27 Gastrointestinal issues occur early in the disorder, are associated with malnutrition and growth failure,28 and are improved by early and aggressive treatment.29 Moreover, the effects of MECP2 mutation on synaptic development are most evident before 2 years of age,30 and targeted treatment options should be administered as early as possible.
In this study, age of diagnosis was associated with clinical, demographic, socioeconomic, and secular factors. Age of classic RTT diagnosis decreased after 2001, possibly due to enhanced developmental screening and widespread implementation of MECP2 testing. Developmental delay, particularly in the motor domain, was associated with earlier diagnosis. However, children who developed and then lost more advanced skills were diagnosed later, as were those with unusual stereotypic hand movements. As in the Australian cohort,7 we found that the age of onset of supportive features was associated with age of diagnosis. Those with less specific features, such as scoliosis, GERD, bone fractures, and self-abusive behaviors often exhibited these features for several years prior to diagnosis. The children of older parents were diagnosed at a younger age, perhaps because experienced parents raised concerns about their children sooner. Although population density was not a factor, higher income was associated with earlier diagnosis. Children with normal head size were typically diagnosed later, perhaps due to the myth that children must have head circumference deceleration to receive the diagnosis; in fact, a substantial proportion do not.10 Alternately, 33% of children with early microcephaly were diagnosed after the median age of diagnosis. Therefore, the message about head circumference is twofold: 1) early acquired microcephaly often goes unrecognized or does not lead to suspicion of RTT, and 2) diagnosis should not be postponed due to the absence of microcephaly.
Although pediatricians diagnosed RTT in a minority of cases, the proportion of pediatricians making the diagnosis has increased since 2006. This increase coincides with publication of the AAP algorithm for developmental surveillance and screening, which focuses on children under age 2 years.9 In general, pediatricians were more likely to diagnose RTT if the child lost the ability to be soothed by their parent or respond to simple commands. Pediatricians were less likely to make the diagnosis when the ability to follow complex commands was lost, or when unusual stereotypies were present. Awareness about the complex nature of both stereotypic behaviors and regression in RTT may improve the likelihood that pediatricians will recognize RTT, refer to community-based resources, and consider sending genetic testing. Pediatricians may elect to refer to a subspecialist prior to suggesting the diagnosis; however, because MECP2 mutations are highly sensitive (although not specific) for RTT, the above findings may prompt genetic testing before referral.
In RTT, children appear developmentally normal during the first 6–18 months of life.2 Most children experience early milestones later than normal,3 and regression occurs after 12 months in over 90%. Therefore children are at risk for late diagnosis due to the “wait-and-see” approach. Delayed diagnosis has been associated with numerous factors, including age of onset of stereotypies, the absence of regression of hand use or verbal language,7 MECP2 mutation type, impaired acquisition of developmental milestones,8 and year of birth.6 In our study, many characteristic features of RTT, including the pathognomonic midline hand stereotypies,31 were present for over a year prior to diagnosis; the diagnosis was often suggested before all criteria were met. Referral and testing based on early features could lead to earlier diagnosis of “probable” RTT1 and targeted treatment during, or even before, the period of regression.
In other disorders, age of diagnosis is associated with similar factors. Both socioeconomic factors32 and abnormal development33 influence age of diagnosis in autism spectrum disorders (ASD). Additionally, those with ASD who would most benefit from therapy are diagnosed later.33 Year of birth34 and number of cooccurring conditions predict age of diagnosis in fragile X syndrome,35 and parents often raise concerns about development over a year before diagnosis.36 Although routine developmental screening in pediatric clinics9 has led to earlier recognition of developmental delay in fragile X syndrome, the age of diagnosis has not changed since 2001 due to the “wait-and-see” approach.35 Genotype and clinical features predict age of diagnosis in neurofibromatosis 2,37 and clinical features and recent year of birth predict earlier diagnosis in Turner syndrome.38 Both are disorders in which timely diagnosis has implications for management. The benefits of early diagnosis include the opportunity for genetic counseling, family planning, decreased psychosocial stress, both increased access to and earlier entry into intervention services, and greater impetus to participate in intervention programs.
Our study suffered some drawbacks, including lack of robust socioeconomic data. We compensated by using estimated measures derived from US census data. Data collection through parental recall could be considered a drawback; however, strong efforts were made to corroborate both diagnosis and age of diagnosis through detailed initial history, complete documentation of MECP2 testing, and thorough review of clinician notes. Examination by a clinician facilitated both collection of retrospective data and comparison with objective clinical features on exam. Moreover, the diagnostic criteria were applied directly by one of the RNHS clinicians.
Conclusion
In this era of emerging targeted therapeutics, early diagnosis is critical. Both recognition by pediatricians and time to diagnosis among subspecialists have improved. However, no systematic effort exists to improve age of diagnosis in Rett syndrome, and median age of recognition among pediatricians has remained stable. The role of pediatricians to recognize early, subtle delay or regression cannot be overemphasized. Diagnosticians successfully recognize most stereotypic behaviors and regression of fundamental skills. However to improve age of diagnosis, physicians should maintain a high index of suspicion between ages 6 months to 3 years, and recognize that concomitant somatic problems and atypical features (e.g., normal head size) can distract from the diagnosis. Greater awareness of specific risk factors for late diagnosis, which include subtle regression and delay in advanced skills, will improve age of diagnosis and care of these complex individuals.
Supplementary Material
Acknowledgements
Drs. Tarquinio and Percy had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Support for design and conduct of the study, as well as collection, management, analysis, and interpretation of the data is provided by grants from the International Rett Syndrome Foundation and from the NIH, including the Angelman, Rett, Prader-Willi syndrome consortium (U54HD61222) a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS) and the Eunice Kennedy Shriver Child Health and Human Development Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Navah Kadish and Karen Pepper for thoughtful review of the manuscript, and Eric Pedrotty for computation of socioeconomic data, all of whom gave written permission for such acknowledgment.
Study Funding: Supported by NIH U54 grants RR019478 (NCRR) and HD061222 (NICHD), and IDDRC grant HD38985 (NICHD), funds from the International Rett Syndrome Foundation and Civitan International Research Center.
Dr. Neul, Ms. Lane, Dr. Glaze, and Dr. Percy are funded by Neuren Pharmaceuticals.
Abbreviations
- RTT
Rett syndrome
- MECP2
Methyl-CpG-binding protein 2 gene
- BMI
Body mass index
- Non-RTT
Participants with a MECP2 mutation who did not meet clinical diagnostic criteria for RTT
- RNHS
Rett syndrome natural history study
- FOC
fronto-occipital head circumference
- IQR
Inter-quartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The remaining authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: No authors have conflicts of interest to disclose.
Contributor’s Statement:
Daniel C. Tarquinio, DO, MS : Dr. Tarquinio participated in study conduct, data collection, manuscript preparation, performed statistical analyses, and approved the final manuscript as submitted.
Wei Hou, PhD : Dr. Hou performed statistical analyses, participated in manuscript preparation, and approved the final manuscript as submitted.
Jeffrey L. Neul, MD, PhD; Jane B. Lane, RN, BSN; Alan K. Percy, MD: Dr. Neul, Ms. Lane, and Dr. Percy participated in study conceptualization, study conduct, data collection, manuscript review, and approved the final manuscript as submitted.
Katherine V. Barnes; Heather M. O’Leary; Walter E. Kaufmann, MD, PhD; Kathleen J. Motil, MD, PhD; Daniel G. Glaze, MD; Steven A. Skinner, MD; Fran Annese, LMSW; Lauren Baggett, MS, CGC; Judy O. Barrish, RN, BSN; Suzanne P. Geerts, MS, RD: Ms. Barnes, Ms. O’Leary, Ms. Bruck, Dr. Kaufmann, Dr. Motil, Dr. Glaze, Dr. Skinner, Ms. Annese, Ms. Baggett, Ms. Barrish, and Ms. Geerts participated in study conduct, data collection, manuscript review, and approved the final manuscript as submitted.
References
- 1.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Annals of neurology. 2010 Dec;68(6):944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg B. Clinical manifestations and stages of Rett syndrome. Mental retardation and developmental disabilities research reviews. 2002;8(2):61–65. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- 3.Neul JL, Lane JB, Lee HS, et al. Developmental delay in Rett syndrome: data from the natural history study. Journal of neurodevelopmental disorders. 2014;6(1):20. doi: 10.1186/1866-1955-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuddapah VA, Pillai RB, Shekar KV, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J Med Genet. 2014 Mar;51(3):152–158. doi: 10.1136/jmedgenet-2013-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neul JL, Fang P, Barrish J, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008 Apr 15;70(16):1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehr S, Bebbington A, Nassar N, et al. Trends in the diagnosis of Rett syndrome in Australia. Pediatric research. 2011 Sep;70(3):313–319. doi: 10.1203/PDR.0b013e3182242461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehr S, Downs J, Bebbington A, Leonard H. Atypical presentations and specific genotypes are associated with a delay in diagnosis in females with Rett syndrome. American journal of medical genetics. Part A. 2010 Oct;152A(10):2535–2542. doi: 10.1002/ajmg.a.33640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehr S, Bebbington A, Ellaway C, Rowe P, Leonard H, Downs J. Altered attainment of developmental milestones influences the age of diagnosis of rett syndrome. Journal of child neurology. 2011 Aug;26(8):980–987. doi: 10.1177/0883073811401396. [DOI] [PubMed] [Google Scholar]
- 9.Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006 Jul;118(1):405–420. doi: 10.1542/peds.2006-1231. [DOI] [PubMed] [Google Scholar]
- 10.Tarquinio DC, Motil KJ, Hou W, et al. Growth failure and outcome in Rett syndrome: specific growth references. Neurology. 2012 Oct 16;79(16):1653–1661. doi: 10.1212/WNL.0b013e31826e9a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2002;6(5):293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Annals of neurology. 1983 Oct;14(4):471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 13.Hagberg B, Goutieres F, Hanefeld F, Rett A, Wilson J. Rett syndrome: criteria for inclusion and exclusion. Brain & development. 1985;7(3):372–373. doi: 10.1016/s0387-7604(85)80048-6. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic criteria for Rett syndrome. The Rett Syndrome Diagnostic Criteria Work Group. Annals of neurology. 1988 Apr;23(4):425–428. doi: 10.1002/ana.410230432. [DOI] [PubMed] [Google Scholar]
- 15.Hagberg B. Clinical delineation of Rett syndrome variants. Neuropediatrics. 1995 Apr;26(2):62. doi: 10.1055/s-2007-979723. [DOI] [PubMed] [Google Scholar]
- 16.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature genetics. 1999 Oct;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 17.Developmental surveillance and screening of infants and young children. Pediatrics. 2001 Jul;108(1):192–196. doi: 10.1542/peds.108.1.192. [DOI] [PubMed] [Google Scholar]
- 18.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998 Feb 28;17(4):407–429. [PubMed] [Google Scholar]
- 19.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992 Jan;89(1):91–97. [PubMed] [Google Scholar]
- 20.Version 21.0. Armonk, NY: IBM Corp; 2012. IBM SPSS Statistics for Windows. [computer program]. [Google Scholar]
- 21.Version 10.1. Redlands, CA: Esri, Inc; 2012. ArcMap Editor. [computer program]. [Google Scholar]
- 22.Version 10.1. Redlands, CA: Esri, Inc; 2014. Address Coder. [computer program]. [Google Scholar]
- 23.Hanks SB, Opitz JM, Reynolds JF. The role of therapy in rett syndrome. American journal of medical genetics. 1986;25(S1):247–252. doi: 10.1002/ajmg.1320250526. [DOI] [PubMed] [Google Scholar]
- 24.Bat-Haee MA. Behavioral training of a young women with Rett syndrome. Perceptual and motor skills. 1994 Feb;78(1):314. doi: 10.2466/pms.1994.78.1.314. [DOI] [PubMed] [Google Scholar]
- 25.Wesecky A, Opitz JM, Reynolds JF. Music therapy for children with rett syndrome. American journal of medical genetics. 1986;25(S1):253–257. doi: 10.1002/ajmg.1320250527. [DOI] [PubMed] [Google Scholar]
- 26.Lotan M. Rett syndrome. Guidelines for individual intervention. The Scientific World Journal. 2006;6:1504–1516. doi: 10.1100/tsw.2006.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotan M. Assistive technology and supplementary treatment for individuals with Rett syndrome. The Scientific World Journal. 2007;7:903–948. doi: 10.1100/tsw.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motil KJ, Caeg E, Barrish JO, et al. Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. Journal of pediatric gastroenterology and nutrition. 2012 Sep;55(3):292–298. doi: 10.1097/MPG.0b013e31824b6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motil KJ, Morrissey M, Caeg E, Barrish JO, Glaze DG. Gastrostomy placement improves height and weight gain in girls with Rett syndrome. Journal of pediatric gastroenterology and nutrition. 2009 Aug;49(2):237–242. doi: 10.1097/MPG.0b013e31818f61fd. [DOI] [PubMed] [Google Scholar]
- 30.Glaze DG. Rett syndrome: of girls and mice--lessons for regression in autism. Mental retardation and developmental disabilities research reviews. 2004;10(2):154–158. doi: 10.1002/mrdd.20030. [DOI] [PubMed] [Google Scholar]
- 31.Nomura Y, Segawa M. Characteristics of motor disturbances of the Rett syndrome. Brain & development. 1990;12(1):27–30. doi: 10.1016/s0387-7604(12)80170-7. [DOI] [PubMed] [Google Scholar]
- 32.Mandell DS, Wiggins LD, Carpenter LA, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Public Health. 2009 Mar;99(3):493–498. doi: 10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry. 2007 Jul;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 34.Bailey DB, Skinner D, Hatton D, Roberts J. Family experiences and factors associated with the diagnosis of fragile X syndrome. J Dev Behav Pediatr. 2000 Oct;21(5):315–321. doi: 10.1097/00004703-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Bailey DB, Jr, Raspa M, Bishop E, Holiday D. No change in the age of diagnosis for fragile×syndrome: findings from a national parent survey. Pediatrics. 2009 Aug;124(2):527–533. doi: 10.1542/peds.2008-2992. [DOI] [PubMed] [Google Scholar]
- 36.Delayed diagnosis of fragile X syndrome--United States, 1990–1999. MMWR Morb Mortal Wkly Rep. 2002 Aug 23;51(33):740–742. [PubMed] [Google Scholar]
- 37.Selvanathan SK, Shenton A, Ferner R, et al. Further genotype--phenotype correlations in neurofibromatosis 2. Clinical genetics. 2010 Feb;77(2):163–170. doi: 10.1111/j.1399-0004.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 38.Massa G, Verlinde F, De Schepper J, et al. Trends in age at diagnosis of Turner syndrome. Archives of disease in childhood. 2005 Mar;90(3):267–268. doi: 10.1136/adc.2004.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.