Abstract
Background & objectives:
One third of the world's population is infected with one or more of the most common soil-transmitted helminths (STH). Albendazole (ALB) is being administered with diethyl carbamazine (DEC) in filariasis endemic areas to eliminate lymphatic filariasis (LF) and helminth infections. In this study, the cumulative impact of seven annual rounds of mass drug administrations (MDA) of DEC and ALB on STH infection in school children in selected villages in southern India was determined.
Methods:
During 2001-2010, seven MDAs were implemented by the Tamil Nadu State Health Department, India. LF and STH infections were monitored in school children from 18 villages of the two treatment arms (viz, DEC alone and DEC+ALB). Kato-Katz cellophane quantitative thick smear technique was employed to estimate STH infections at three weeks, six months and one year post MDA.
Results:
Prior to treatment, an overall STH prevalence was 60 per cent. After each MDA, infection markedly reduced at three weeks post-treatment in both the arms. The prevalence increased at six months period, which was maintained up to one year. After seven rounds of MDA, the infection reduced from 60.44 to 12.48 per cent in DEC+ALB arm; while the reduction was negligible in DEC alone arm (58.77 to 52.70%).
Interpretation & conclusions:
Seven rounds of MDA with DEC+ALB reduced the infection load significantly, and further sustained low level of infection for 10 years. However, complete parasite elimination could not be achieved. To curtail STH infection in the community, MDA should be regularized and environmental sanitation measures need to be improved by effective community-based campaigns.
Keywords: albendazole, children, diethylcarbamazine, mass drug administration, soil-transmitted helminth
According to WHO, more than 1.5 billion people or 24 per cent of the world's population are infected with soil-transmitted helminths (STH). Widely distributed globally in tropical and subtropical areas, most infections occur in sub-Saharan Africa, the Americas, China, and East Asia. Over 270 million preschool-age children and over 600 million school-age children live in areas where STH is transmitted1.
Morbidity due to STH was estimated to be 39 million, or almost 8 per cent of the disease burden due to infectious diseases2. One third of the world's population is infected with one or more of the most common STH the roundworm, Ascaris lumbricoides; the hookworms, Ancylostoma duodenale and Necator americanus; and the whipworm, Trichuris trichiura3. The best evidence on costs and benefits of STH deworming relates to school-age children, since they are easy to reach en masse through the school system, making it a low-cost intervention, while treatment of this group substantially reduces morbidity, improves growth, and helps in improving educational performances.
In 1997, the Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched in response to a specific resolution by the World Health Assembly4. The main strategy in the Programme is to interrupt transmission by annual treatment with antifilarial drugs [diethyl carbamazine (DEC) or ivermectin plus albendazole (ALB)]. In addition, morbidity management reduces the suffering of patients who have chronic manifestations. The GPELF has targeted elimination of LF by 2020 globally, and this Programme has an unprecedented public health impact on both LF and other neglected tropical diseases5. As part of the Programme for elimination of lymphatic filariasis, antihelminthic drug albendazole, is being administered along with DEC in the filariasis endemic areas, and to provide additional antifilarial efficacy along with salutary effects on intestinal helminth infections6,7,8. This study was undertaken to evaluate the impact of seven annual rounds of mass drug administration with DEC alone and in combination with ALB in primary school children on prevalence of STH in the selected 18 villages of two blocks in Tamil Nadu, south India.
Material & Methods
Study area: Two revenue blocks, viz. Tirukoilur and Mugaiyur, located in the Villupuram district of Tamil Nadu State, India, endemic for bancroftian filariasis caused by Wuchereria bancrofti, were chosen for the study. The study area covered a population of 321,000, which included about 100,000 children. Most of the houses had mud walls and thatched roofs.
Selection criterion of villages and children: In order to have baseline data for the two blocks, 24 villages from Tirukoilur and 27 villages from Mugaiyur (total of 51 villages) were screened and nine index villages were selected from each block based on population size of the villages. The survey on geohelminths was conducted in these 18 index villages by including all 18 primary schools in the area, whose students had never been treated against helminths by any community-based programme, prior to the present study. Within a given school, children aged 9-10 yr were selected by a simple random sampling technique, to draw the necessary numbers (20-50) from children of that age group, as described earlier9. The World Health Organization guidelines10 were followed to assess the prevalence of geohelminthiasis, species profile and intensity. As per the guidelines, the age group 9-10 yr was the ideal target group, and a number of 200-250 individuals was an adequate sample for each ecologically homogeneous area to evaluate prevalence and intensity of STH infection.
Mass drug administration (MDA): From March 2001, Tirukoilur block was mass administered with annual single dose of antifilarial drugs viz. DEC +ALB (DEC- 6 mg/kg; ALB – 400mg), while DEC alone in Mugaiyur block. The drugs were distributed in March 2001, March 2002, March 2003, September 2004, December 2007, June 2009 and February 2010. The peripheral health workers managed the mild side effects reported by the individuals complied with the drug administration. Seven rounds of MDA were implemented in each of the two blocks.
Impact assessment of MDA: Before initiating the survey, a meeting was organized among the parents and teachers of class IV and V students, to explain the purpose of the study and to include their children in the study. A total of 320 to 500 school children were screened from each arm during each observation. The prevalence and intensities of STHs viz. round worm, hookworms and whipworm were estimated by using Kato-Katz cellophane quantitative thick smear technique9. The survey was carried out at three weeks, six months and one year post MDAs. The study, as part of the long term project was cleared by the institutional ethical committee of Centre for Research in Medical Entomology, Madurai.
Indicators measured: Prevalence of the infections was expressed as percentage of subjects found positive for helminths of a particular species or in combination in each survey. The per cent prevalence reduction was computed as follows;
% prevalence reduction at time “t” =
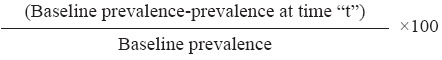
Intensity of STHs infection was reported as geometric mean intensity (GMI)11,12 of eggs per gram.
% prevalence reduction at time “t” =
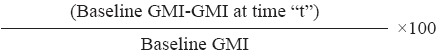
Statistical analysis: The data were analyzed using the statistical package SPSS/PC+, version 10 USA. For prevalences, 95% confidence limits were calculated. Chi-square test was used to test for prevalence difference between arms at different time periods. Using univariate analysis, odds ratios (ORs) including 95% confidence intervals (CIs) were calculated for age and sex. The egg counts of A. lumbricoides, T. Trichiura and hookworm were examined for normality by Kolmogorov-Smirnov test, and were not observed to be normally distributed, therefore, the assessment of the variation of egg counts was done after log transformation. Student t test was used to compare means of log values of egg counts between two arms at different time points.
Results
Baseline survey was carried out in March 2001, prior to the annual MDA programme. In this survey, stool samples from 321 (50% coverage) and 325 (39% coverage) school children of age group 9-10 yr were analysed in Tirukoilur (DEC+ALB) and Mugaiyur (DEC alone) arms, respectively. It was observed that 60.4 per cent (95% CI: 55.09, 65.79) and 58.8 per cent (95% CI: 53.42, 64.12) of the students harboured any one of the three intestinal worms in the two treatment arms, respectively (Table I). There was no significant difference in the prevalence of infection between the villages. All the villages had similar ecological characteristics and the children were equally exposed to soil transmitted helminth infections in both the arms. Among the infected, double infection was observed in 51 children (26.3%) in DEC+ALB arm; while 32 children (16.8%) in DEC alone arm and the difference was significant (P<0.05). Triple infection (i.e. infection with all the 3 helminths) was found in one and two children in the two arms, respectively. Roundworm was the most common helminth observed with a prevalence of 54.8 and 52.9 per cent, followed by hookworm (16.5 and 8.3%) and whipworm with a low prevalence of 4.7 and 6.8 per cent in the DEC+ALB arm, and DEC alone arm respectively (Table II). The baseline prevalence (March 2001) in both the treatment arms was not statistically different.
Table I.
Prevalence and percentage reduction of different STHs infection three weeks post (MDA) mass drug administration periods
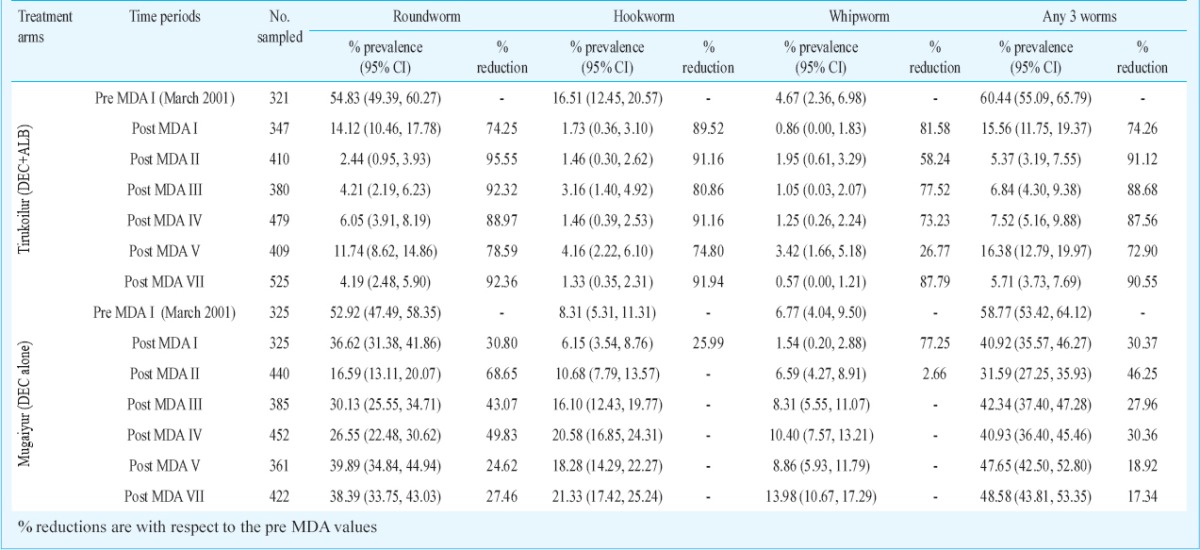
Table II.
Percentage change in the prevalence and intensity of STHs after seven rounds of mass drug administration (MDA)
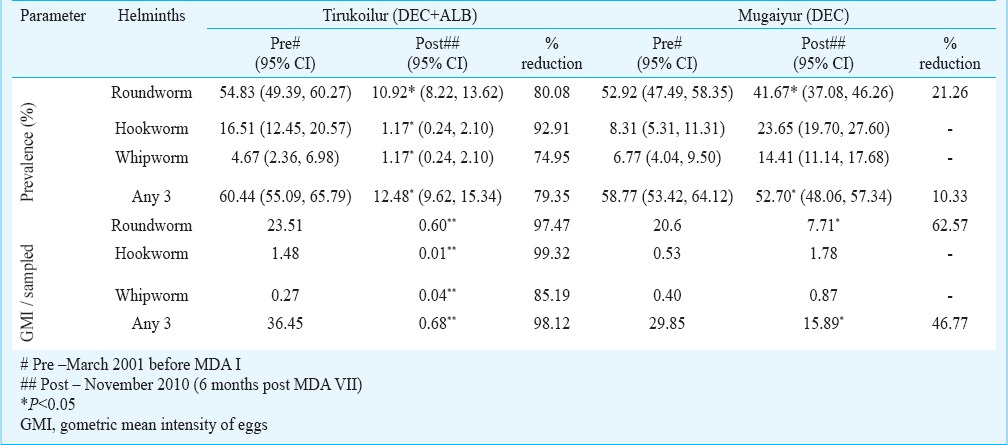
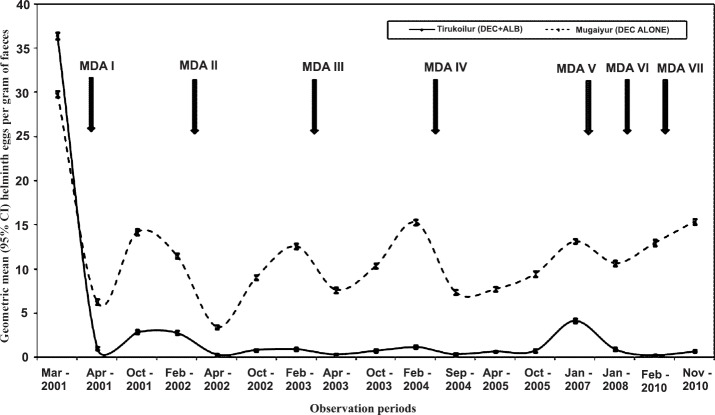
Impact evaluation at different time points with respect to infection prevalence in the two arms: Overall there were 17 observations carried out between the years 2001 and 2010 during pre- and post- MDA. In Tirukoilur Block where combination of DEC and ALB was administered, there was a sudden fall of STH prevalence immediately after the first MDA in 2001 (60.4 to 15.6% with a reduction of 74%) during three weeks post MDA (Fig. 1). Subsequently at six months post-treatment, the prevalence doubled to 30.7 per cent which was maintained for one year period. After the 2nd MDA also, a steep fall in prevalence was observed at three weeks (82% reduction compared to pre MDA II value), which regained to 13 per cent level. In the subsequent MDAs, the reduction during post three week MDAs were <50 per cent and the prevalence was maintained at 6-15 per cent level. During the final observation of this study (i.e. 6 months post-MDA VII), the STH prevalence was 12.5 per cent (95% CI: 9.62 -15.34%) (Table II). The overall prevalence declined from 60.4 to 12.5 per cent with a percentage reduction of 79. Among the three helminths analysed, highest reduction after seven rounds of MDA was observed for hookworm (92.9%), followed by roundworm (80.1%) and whipworm (74.9%) (Table II).
Fig. 1.
Prevalence (%) of soil-transmitted helminths (STHs) in two different treatment arms during different time-points with mass drug administration (MDA).
In the DEC alone arm (Mugaiyur Block), the decline in prevalence rates with MDA was negligible (Fig. 1), and at three weeks post MDA I and II, the reductions observed were 31 and 39 per cent, respectively. But at 12 months post MDA, the values were observed to reach pre-treatment level. These reductions were mainly attributed to the decline in A. lumbricoides. In this arm, infection due to all the helminths declined during the post MDA I period, while in subsequent rounds of MDA A. lumbricoides alone was reduced. The prevalence rates for roundworm, hookworm and whipworm were 54.8, 16.5 and 4.7 per cent during pre-MDAs, which reduced to 10.9, 1.2 and 1.2 per cent, respectively at post MDA VII (Table II). The cumulative prevalence rate declined from 58.8 per cent (95% CI: 53.42- 64.12%) to 52.7 per cent (95% CI: 48.06- 57.34%) after seven rounds of MDA. Among the three helminths, reduction was observed for A. lumbricoides (21.3%) alone.
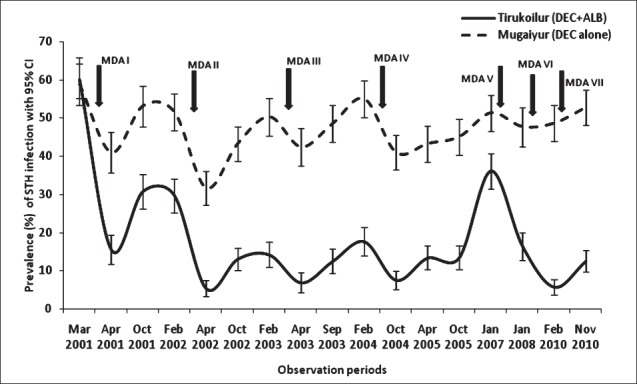
For any of the three worms, the percentage reduction in prevalence was 7.7 times higher in 2-drug combination arm (DEC+ALB) than 1-drug arm. The re-infection rates after each MDA was estimated, by considering the 11 months post-treatment values. It was found that the re-infection was higher (>80%) in DEC alone arm than in the DEC+ALB arm (50%), for the initial two rounds of MDA (Table III). Subsequently the rates were observed to be similar in both the treatment arms.
Table III.
Residual infection rates in % of helminths with treatment using diethyl carbamazine (DEC) with and without albendazole (ALB)
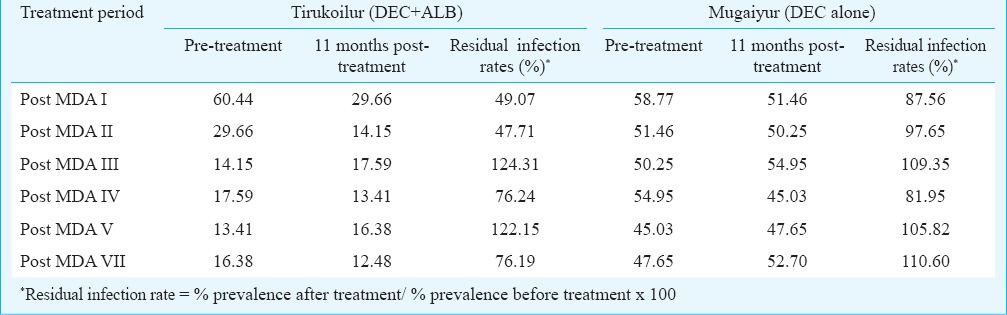
As the effect on A. lumbricoides was significant, the relative changes in prevalence for this helminth in both the treatment arms was analysed at three weeks post-MDA (Fig. 2). The prevalence in both the arms was comparable during the pre-MDA period, while after MDA I the values reduced to 14.1 and 36.6 per cent, which further declined to 2 and 17 per cent, respectively in Tirukoilur and Mugaiyur arms during post-MDA II. In the subsequent rounds of MDA, the prevalence was maintained at 4-6 per cent in Tirukoilur arm (DEC+ALB), while at about 30 per cent in Mugaiyur arm (DEC alone).
Fig. 2.
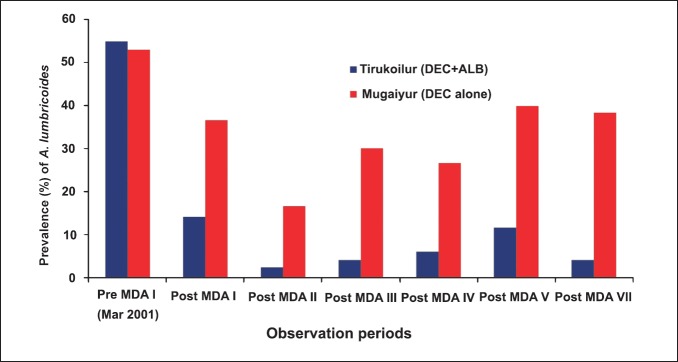
Prevalence of A. lumbricoides during three weeks post MDAs in the two treatment arms.
Co-infection of helminth infections: Co-infection of two helminths in children was observed at various time points, and A. lumbricoides was observed to be predominant in most of the children with double infection. Therefore, the association of A. lumbricoides with other two helminths was analysed. Ascaris lumbricoides was found to occur more with hookworm during different time points (>40%) rather than with T. trichiura (<30%) (Fig. 3). The prevalence of A. lumbricoides co-infection with hookworm was almost double than that with whipworm at most of the time periods. During the last observation (i.e. post-MDA VII), dual infection of hookworm with T. trichiura was not observed in any of the infected children in DEC+ALB arm; while 22.1 per cent of children in DEC alone arm were found with this co-infection.
Fig. 3.
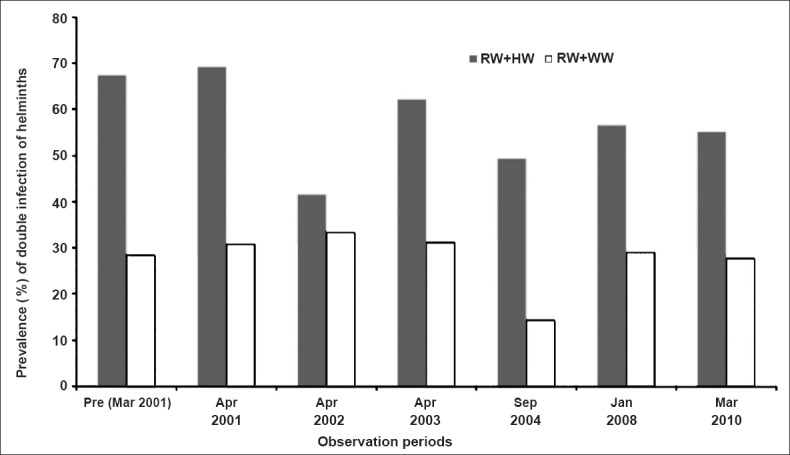
Association between Ascaris and other two helminth infections in school children. RW, roundworm, WW, whipworm; HW, hookworm.
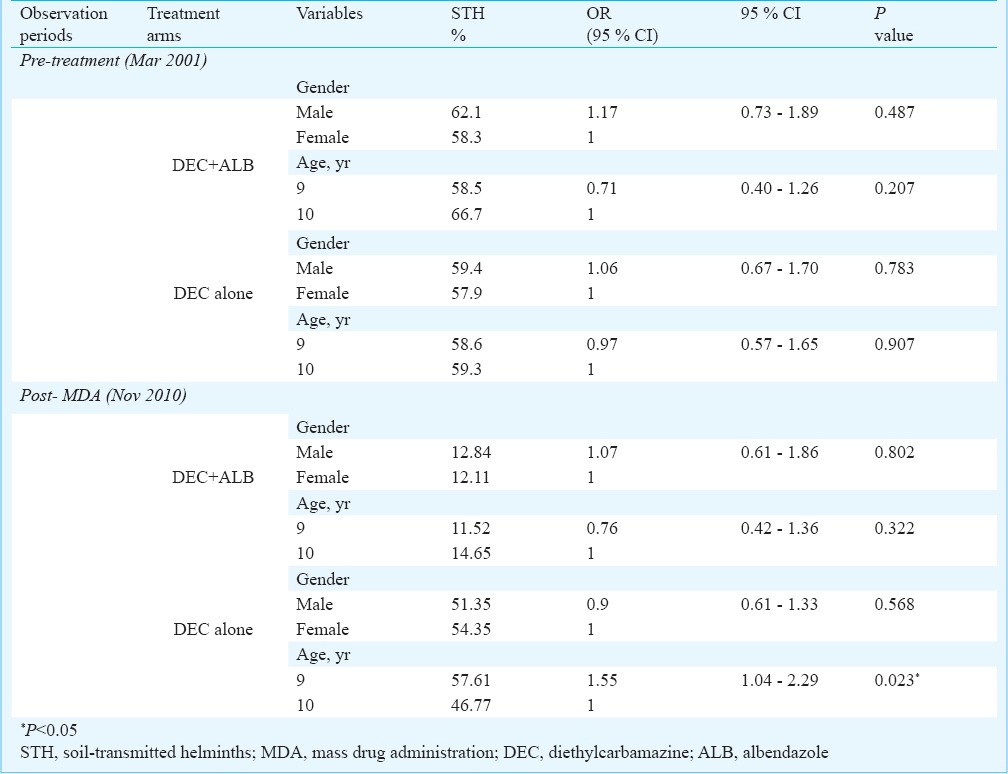
Risk assessment for age and gender and comparison of risk between arms: The results on the effect of age and gender on helminth infection in school children in the two treatment arms before and after MDA are shown in Table IV. During the baseline survey no significant association of helminth infection with sex or age was observed in both the treatment arms. However, during the post-treatment, nine years children in DEC alone arm were at 1.6-fold higher risk for helminth infection than 10 yr children (OR = 1.55, 95% CI: 1.04, 2.29; P < 0.05).
Table IV.
Association of exposure and infection with STH from two treatment arms before and after MDA
Baseline intensity of eggs: The geometric mean egg intensity (eggs per gram of faeces) of any of the three helminths was 36.45 and 29.85 during the baseline survey (in March 2001) in DEC+ALB and DEC alone arms, respectively (Table II). There was no significant difference in the mean egg intensity, village-wise. A. lumbricoides formed the major helminth among the three in both the treatment arms (GMI was 23.5 and 20.6 in the two arms, respectively).
Impact evaluation at different time periods with respect to egg intensity in two arms: In DEC+ALB co-administered arm, there was 97 per cent reduction in epg (eggs/g) values immediately after MDA I (36.45 reduced to 2.78) which was maintained for 11 months (Fig. 4). After each MDA, the mean egg reductions post three weeks were always >97 per cent. For any of the three worms the GMI was 0.68 at 6 months post MDA VII (last observation in this study). The overall decline in the egg intensity [expressed as eggs per gram (epg) of faeces] after seven rouds of MDA, in this arm was 98% (P <0.05). For individual helminth, the overall reductions were 97, 99 and 85 per cent for round worm, hookworm and whipworm, respectively (Table II).
Fig. 4.
Egg intensity of STH in school children before and after mass drug administration.
In DEC alone arm, the immediate reduction in egg intensity was 79 per cent, which became 62 per cent (29.85 reduced to 11.47) at 11 months post MDA (Fig. 4). During subsequent MDAs there were negligible changes in the egg intensity. During three weeks post MDA, the GMI reductions ranged from 57 to 88 per cent. The GMI (epg) value for any of the three worms was 15.89 at six months post-MDA VII (Table II). For individual helminth, the reduction in DEC alone arm was observed only for A. lumbricoides. The decline after seven rounds of MDA for any three helminth was 47 per cent (P <0.05) in this arm.
Discussion
Co-administration of DEC with ALB in filariasis elimination programme has necessitated the need to evaluate the relative and the combined role of these drugs on other parasitic infections. Combination of ALB with DEC had demonstrated significant cure rates of 74.3 per cent and egg reduction rates of 97.3 per cent after three weeks of treatment, and the benefit was observed to be 2.5 times more than with DEC drug alone12. The drug efficacy was sustained even after 11 months post-treatment in the ALB combination arm. The cure rates were 4-times higher in this arm for any of the three intestinal worms. In a longitudinal study, 20 times higher cure rates were observed for any three worms in ALB co-administration arm (40% against 2% in DEC alone) after 11 months post MDA 200113
For the last 10 years period (2000-2010), seven rounds of MDA were implemented as part of the National Programme for Elimination of Lymphatic Filariasis in Tamil Nadu. In the study area, A. lumbricoides was most common than the other two helminths. In West Malaysia, the overall prevalence of STH infection was 73.2 per cent with T. trichiura infection being the most predominant (66.8%) followed by A. lumbricoides (38.5%) and hookworm (12.8%)14. In the present study, the trend of infection levels for the three STH species were A. lumbricoides>hookworm>T. trichiura. Although hookworm can be transmitted by ingesting contaminated food with larva, these mainly infect by penetrating the host's skin when the host makes contact with soil. In Malaysia, all the age groups were included in the study (ranging from 1 to 83 yr with a median age of 11 yr), which could have enhanced the overall prevalence rates, as the present investigation was confined to the age group of 9-10 yr. In our study A. lumbricoides was found to be more associated with hookworm during different time points rather than with T. trichiura. But, in Malaysia14 and Zanzibar15 the combination of T. trichiura and A. lumbricoides was the predominant co-infection observed.
In the present study, during post MDA I there was 74 and 30 per cent reduction in the overall helminths in DEC+ALB and DEC alone arms, respectively. The prevalence reduction in DEC alone arm was mainly attributed to the decline in A. lumbricoides infections. With seven rounds of MDA, these reductions were sustained, and there was significant decrease in the prevalence and egg intensities of individual helminth in the arm with the combination of DEC and albendazole, but not in DEC alone arm. In DEC alone arm the re-infection of A. lumbricoides alone could be prevented.
In Haiti, ALB either alone or in combination with DEC reduced the prevalence of A. lumbricoides, T. trichiura and hookworm16. Our study did not have ALB alone arm, but such arms were available in other studies17,18. Administration of annual dose of diethylcarbamazine citrate and albendazole is currently advocated by WHO for control of LF in several endemic countries, since this combination is more effective than either drug administered alone19. It was observed that ALB alone was not effective in the complete removal of the STH parasites from a population. The cure rates with a single dose of ALB were 88, 78 and 28 per cent for roundworms, hookworms and whipworm, respectively20. The lower impact on whipworms was attributed to their location in the lower intestines. The combination of ALB+IVR (ivermectin) arm was found to be more effective against whipworms17 and produced higher cure rates for this helminth as compared to DEC+ALB. Our study showed sustained efficacy against all three STHs for DEC+ALB co-administration for 10 years period.
For effective de-worming, repeated treatment bi-annually for 1-2 years with single dose antihelminthics has been recommended21. Our results showed that the frequency could be reduced to yearly basis using two drug combination of DEC and ALB. In the present study, when MDA was not done for two years (2005 and 2006), an increase in prevalence (36%) and egg intensity (GMI=4.15) was observed in the 2-drug arm, as compared to other periods, where the yearly treatment interval was maintained. Therefore, the consistency in the treatment interval should be complied in the control programme.
There are limited studies comparing the efficacy of 2-drug combinations of DEC+ALB with other drugs for intestinal helminth control9,12,17,22. In a field-based three-arm clinical trial in Kenya22, 67.4, 71.1 and 78.8 per cent cure rates for STHs were reported among persons treated with DEC, ALB and a combination of DEC+ALB, respectively after six months of single-dose treatment. Our study showed that the prevalence observed during the six months post-treatment period did not significantly vary even after one year. This combination was well tolerated and efficacious in reducing intestinal helminth infection and also resulted in improved weight gain in T. trichiura infected children17. With DEC alone there was 17.1, 21.6 per cent and nil reductions for A. lumbricoides, hookworm and T. trichiura, respectively after five weeks post-treatment16. In the present study, decline in prevalence rates was observed for hookworm and T. trichiura post MDA I, which did not reduce further during subsequent rounds of MDA Children not attending school or those who had missed the treatment, together with preschool children might contribute substantially to the overall transmission of STH. Higher risk of helminth infection (mainly hookworm and T. trichiura) in DEC alone arm after seven rounds of MDA, might be due to the reduced drug impact on these helminths. A 6-year cross-sectional follow up study in two villages of Northern Tanzania showed a reduction in both the prevalence and intensity of infection with roundworm and whipworm, but not for hookworm, and it was suggested that the reduction was likely to be the result of health related intervention rather than drug administration23.
In conclusion, the present results showed that after 10 years of repeated annual rounds of MDA, the transmission indices reached negligible levels in both the treatment arms. Significant reduction was observed in the prevalence and infection intensity by >80 per cent for each STH infection in the present study. However, complete elimination of the parasite from the community could not be achieved. More studies are required to determine the optimum rounds (annually) of drug administration (with a combination of DEC +ALB), essential for complete cessation of STH transmission. The implementation of complementary measures such as improved environmental sanitation and hygiene, which aim to improve factors that contribute to the transmission of STHs is essential to consolidate the gains achieved by the MDA.
Acknowledgment
The authors acknowledge the financial support received from WHO/TDR, Geneva (ID No. A00257). The authors thank Dr. P. Vanamail, All India Institute of Medical Sciences, New Delhi, for his valuable suggestions during data analysis, and acknowledge the technical assistance of staff members of CRME's Tirukoilur field station.
References
- 1.WHO Media centre, Soil-transmitted helminth infections, Fact sheet N°366 Updated April 2014. [accessed on December 18, 2014]. Available from: http://wwwwhoint/mediacentre/factsheets/fs366/en/
- 2.Hotez PJ, Molyneux DH, Fenwick A, Molyneux D. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson RD. An update on the geohelminths: Ascaris lumbricoides, Hookworms, Trichuris trichiura, and Strongyloides stercoralis. Curr Infect Dis Rep. 2002;4:59–64. doi: 10.1007/s11908-002-0068-1. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux DH, Zagaria N. Lymphatic filariasis elimination: progress in global programme development. Ann Trop Med Parasitol. 2002;96(Suppl):15–40. doi: 10.1179/000349802125002374. [DOI] [PubMed] [Google Scholar]
- 5.Ottesen EA, Hooper PJ, Bradley M, Biswas G. The Global Programme to Eliminate Lymphatic Filariasis: Health Impact after 8 Years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horton j, Witt C, Ottesen EA, Lazdins JK, Addiss DJ, Awadzi K, et al. An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis. Parasitology. 2000;121(Suppl):S147–60. doi: 10.1017/s0031182000007423. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux DH, Neira M, Liese B, Heymann D. Lymphatic filariasis: setting the scene for elimination. Trans R Soc Trop Med Hyg. 2000;94:589–91. doi: 10.1016/s0035-9203(00)90198-6. [DOI] [PubMed] [Google Scholar]
- 8.Ottesen EA. The global programme to eliminate lymphatic filariasis. Trop Med Int Health. 2000;5:591–4. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- 9.Mani TR, Rajendran R, Sunish IP, Munirathinam A, Arunachalam N, Satyanarayana K, et al. Effectiveness of two annual single-dose mass drug administrations of diethylcarbamazine alone or in combination with albendazole on soil-transmitted helminthiasis in filariasis elimination programme. Trop Med Int Health. 2004;9:1030–5. doi: 10.1111/j.1365-3156.2004.01298.x. [DOI] [PubMed] [Google Scholar]
- 10.Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Unpublished document WHO/CTD/SIP/98.1. Geneva: World Health Organization; 1998. [accessed on April 15, 2012]. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes. Available from: http://whqlibdoc.who.int/hq/1998/who_ctd_sip_98.1.pdf . [Google Scholar]
- 11.Kightlinger LK, Seed JR, Kightlinger MB. The epidemiology of Ascaris lumbricoides, Trichuris trichiura and hookworms in children in the Ranomafana rainforest, Madagascar. J Parasitol. 1995;81:159–69. [PubMed] [Google Scholar]
- 12.Mani TR, Rajendran R, Munirathinam A, Sunish IP, Abdullah SM, Augustin DJ, et al. Efficacy of co-administration of albendazole and diethylcarbamazine on geohelminthiases: a study from South India. Trop Med Int Health. 2002;7:541–8. doi: 10.1046/j.1365-3156.2002.00894.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R, Mani TR, Munirathinam A, Sunish IP, Abdullah SM, Augustin DJ, et al. Sustainability of soil-transmitted helminth control following a single-dose co-administration of albendazole and diethylcarbamazine. Trans R Soc Trop Med Hyg. 2003;97:355–9. doi: 10.1016/s0035-9203(03)90168-4. [DOI] [PubMed] [Google Scholar]
- 14.Ngui R, Ishak S, Chuen CS, Mahmud R, Lim YAL. Prevalence and risk factors of intestinal parasitism in rural and remote West Malaysia. PLoS. Negl Trop Dis. 2011;5:e974. doi: 10.1371/journal.pntd.0000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopp S, Mohammed KA, Rollinson D, Stothard JR, Khamis S, Utzinger J, et al. Changing patterns of soil-transmitted helminthiases in Zanzibar in the context of National Helminth Control Programs. Am J Trop Med Hyg. 2009;81:1071–8. doi: 10.4269/ajtmh.2009.09-0377. [DOI] [PubMed] [Google Scholar]
- 16.Fox LM, Furness BW, Haser JK, Desire D, Brissau JM, Milord MD, et al. Tolerance and efficacy of combined diethylcarbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. Am J Trop Med Hyg. 2005;73:115–21. [PubMed] [Google Scholar]
- 17.Ismail MM, Jayakody RL. Efficacy of albendazole and its combinations with ivermectin or diethylcarbamazine (DEC) in the treatment of Trichuris trichiura infections in Sri Lanka. Ann Trop Med Parasitol. 1999;93:501–4. doi: 10.1080/00034989958230. [DOI] [PubMed] [Google Scholar]
- 18.Beach MJ, Streit TG, Addiss DG, Prospere R, Roberts JM, Lammie PJ. Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian school children. Am J Trop Med Hyg. 1999;0 60:479–86. doi: 10.4269/ajtmh.1999.60.479. [DOI] [PubMed] [Google Scholar]
- 19.Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today. 1999;15:382–6. doi: 10.1016/s0169-4758(99)01486-6. [DOI] [PubMed] [Google Scholar]
- 20.Keiser J, Utzinger J. Efficacy of current drugs against soil transmitted helminth infections: systematic review and meta-analysis. J Am Med Assoc. 2008;299:1937–48. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 21.Ananthakrishnan S, Das PK. Integrated programme for control of geohelminths: A perspective. Natl Med J India. 2001;14:148–53. [PubMed] [Google Scholar]
- 22.Njenga SM, Gatika SM, Mbu J, Wamme CN. Comparative efficacy of diethylcarbamazine, albendazole and a combination of diethylcarbamazine and albendazole in clearance of multiple helminth infections, Kwale District, Kenya. Am J Trop Med Hyg. 1999;61(Suppl):S444–5. [Google Scholar]
- 23.Poggensee G, Krantz I, Nordin P, Mtweve S, Ahlberg B, Mosha G, et al. A six year follow-up of school children for urinary and intestinal schistosomiasis and soil-transmitted helminthiasis in Northern Tanzania. Acta Trop. 2005;93:131–40. doi: 10.1016/j.actatropica.2004.10.003. [DOI] [PubMed] [Google Scholar]