FIG 5.
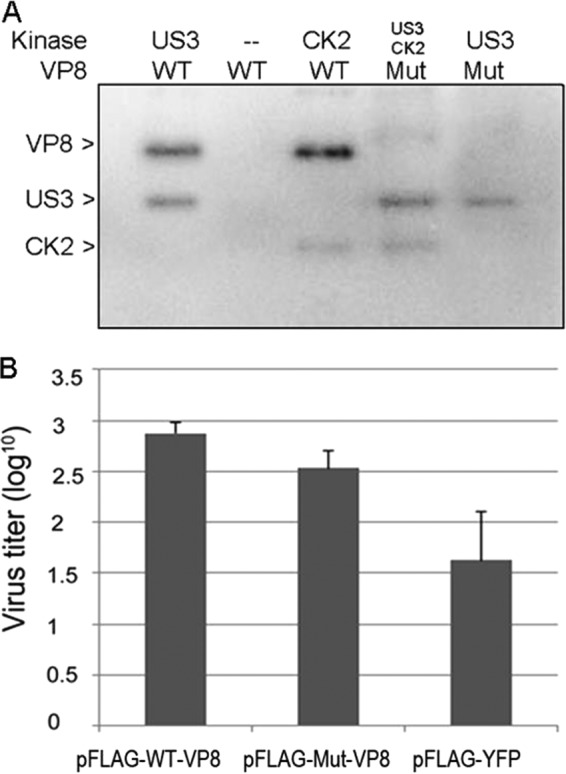
WT-VP8 benefits virus replication more than Mut-VP8, which is not phosphorylated by CK2 and US3. (A) Confirmation of nonphosphorylated Mut-VP8 in the in vitro kinase assay. Mut-VP8, which had all the critical phosphorylation sites for US3 (S16) and for CK2 (T65, S66, S79, S80, S82, S88, and T107) replaced by alanines, was constructed and analyzed in kinase assays with CK2 and US3. (B) BoHV-1 ΔUL47 replication in WT-VP8-expressing cells and in Mut-VP8-expressing cells. FBT cells were infected with BoHV-1 ΔUL47 at a multiplicity of infection of 0.3. At 4 hpi, cells were transfected with pFLAG-WT-VP8 or pFLAG-Mut-VP8. A control sample was transfected with pFLAG-YFP. Viruses were collected at 36 hpi and titrated on MDBK cells.
