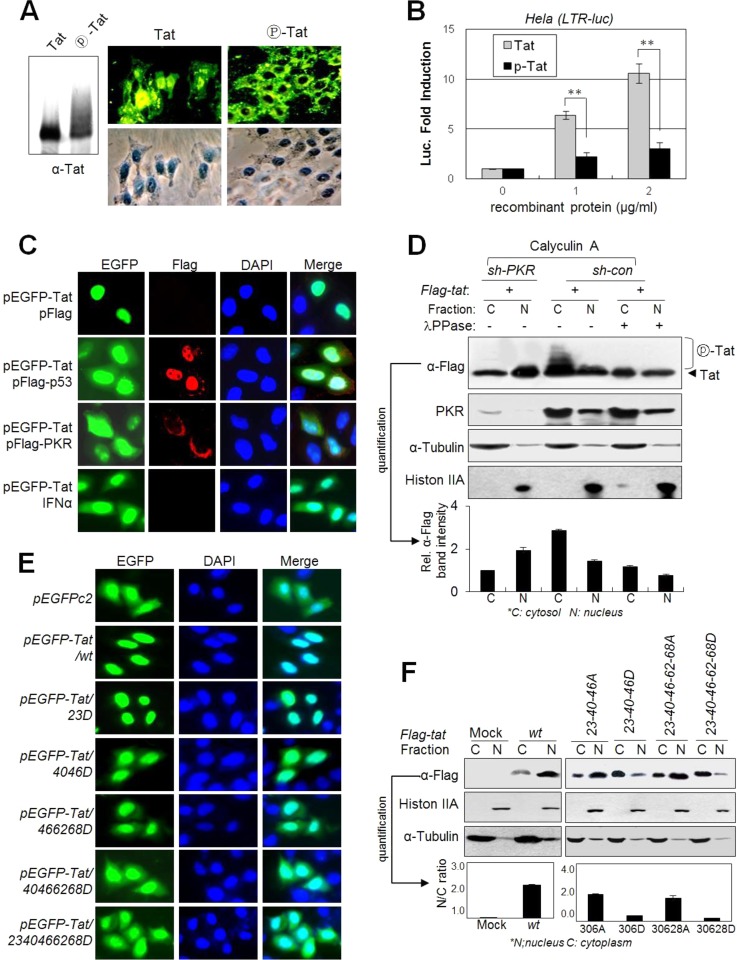
FIG 6.
PKR-mediated Tat phosphorylation blocks Tat nuclear localization. (A) Phospho-free normal Tat and phospho-Tat (fully phosphorylated by PKR for 4 h) recombinant proteins were assessed by Western blotting (left panel) with anti-Tat Ab. HeLa cell monolayers were incubated with 1 μg/ml normal or fully phosphorylated Tat proteins in DMEM. After 1 h of incubation, cells were washed and gently trypsinized (1× trypsin-EDTA) for 2 min at 37°C to remove membrane-bound unpenetrated Tat protein. Intracellular Tat was assessed by immunocytochemistry with anti-Tat and FITC-conjugated secondary antibodies. (B) HeLa cells transfected with pLTR-luc (200 ng) and pCMV-lacZ (20 ng) plasmids were treated with increasing amounts of normal Tat and phospho-Tat proteins (0 to 2 μg/ml). After 24 h, luciferase activity was assessed and is represented as means ± SD (n = 3). *, P < 0.01. (C) pEGFP-tat-transfected HeLa cells were cotransfected with pcDNA3-Flag-p53 or pcDNA3-Flag-PKR or treated with 1,000 U/ml of IFN-α. Twenty-four hours after transfection, cells were photographed under a fluorescence microscope (magnification, ×400). (D) PKR-normal (sh-con) and PKRKD (sh-PKR) HEK293 cells were transfected with pFlag-tat. Two days after transfection, cells were treated with 50 nM calyculin A for 1 h prior to harvest, and then nuclear and cytosolic fractions from the cell extracts were prepared using the NER/PER kit (Pierce) and assessed by Western blotting. Shifted Tat bands are indicated. Relative intensities of Tat bands in the Western blot are represented as means ± SEM (n = 3) after quantification three times using TINA 2.0 software (Raytest, Straubenhardt, Germany). (E) HeLa cells were transfected with GFP-conjugated wt or D-mt Tat plasmids. Two days after transfection, cells were fixed and photographed under a fluorescence microscope (magnification, ×200). (F) HEK293 cells were transfected with Flag-tagged wt or mt Tat plasmids as indicated. Nuclear and cytosolic fractions from the cell extracts were assessed by Western blotting with appropriate antibodies. Relative intensities of the Tat bands in the cytoplasm and the nucleus are represented.