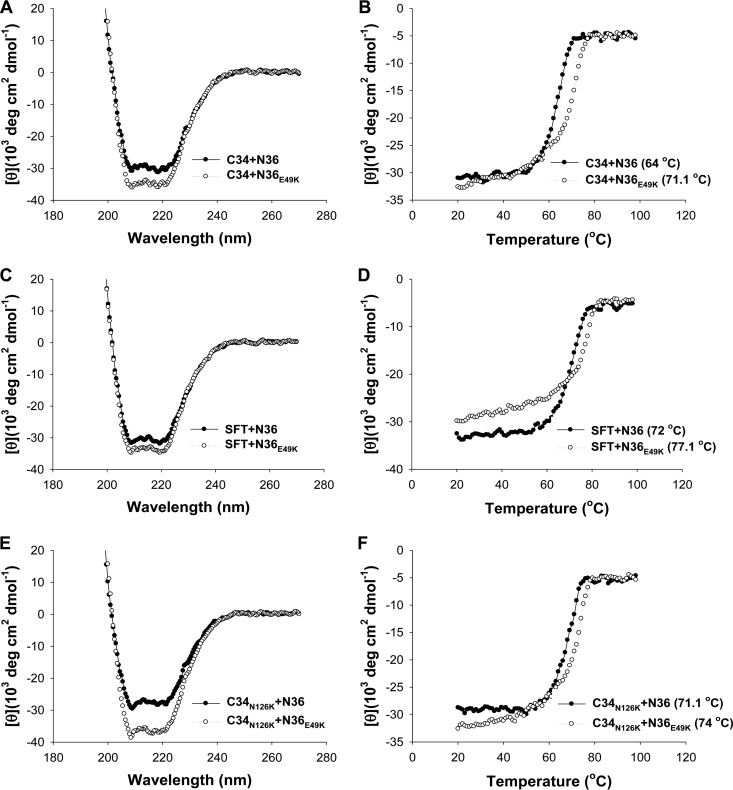
FIG 5.
Binding stability of C34, SFT, and a C34 mutant determined by CD spectroscopy. The α-helicity and thermostability of 6-HBs formed by C34 (A and B) or SFT (C and D) with N36 or N36E49K were measured. The Tm value was defined as the midpoint of the thermal unfolding transition. The final concentration of each peptide in PBS is 10 μM. The binding stability of C34 carrying an N126K mutation (C34N126K) with N36 or N36E49K was similarly determined by CD spectroscopy (E and F). The experiments were repeated at least three times, and representative data are shown.