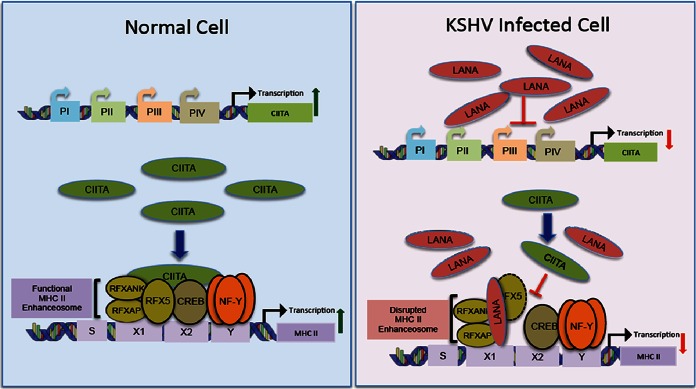
FIG 10.
Proposed model of MHC-II transcriptional downregulation through interactions of LANA with RFX proteins. MHC-II expression is regulated predominantly at the transcriptional level, and transcription of MCH-II genes is strictly contingent upon the binding of a non-DNA-binding transactivator, CIITA, to the promoters of MHC-II genes. The promoters of all the MHC-II genes contain a highly conserved cis-acting module that consists of the S-, X1-, X2-, and Y-box sequences. A heterotrimeric RFX complex consisting of RFX5, RFXAP, and RFXANK occupies the X1 box. The X2 and Y boxes are occupied by CREB protein and trimeric NF-Y complexes, respectively. Proper assembly of these proteins on MHC-II promoters makes a functional MHC-II enhanceosome. In normal cells, the assembled MHC-II enhanceosome initiates the transcription of MHC-II genes by recruiting CIITA to the promoters of MHC-II genes. However, in KSHV-infected cells, the KSHV latent protein LANA, as shown previously, reduces the transcriptional activity of CIITA promoters PIII and PIV, resulting in reduced expression of CIITA, which in turn contributes to an inhibition of MHC-II genes. There is an additional mechanism to inhibit the binding of residual CIITA to the promoters of MHC-II. The results of the present study show that LANA binds to the components of the RFX complex at MHC-II promoters and disrupts the assembly of these factors. The disrupted RFX complex is unable to recruit CIITA and thus mitigates the efficiency of residual CIITA in stimulating MHC-II transcription. The culminating effect of these pathways leads to a reduction in the transcription of MHC-II genes.