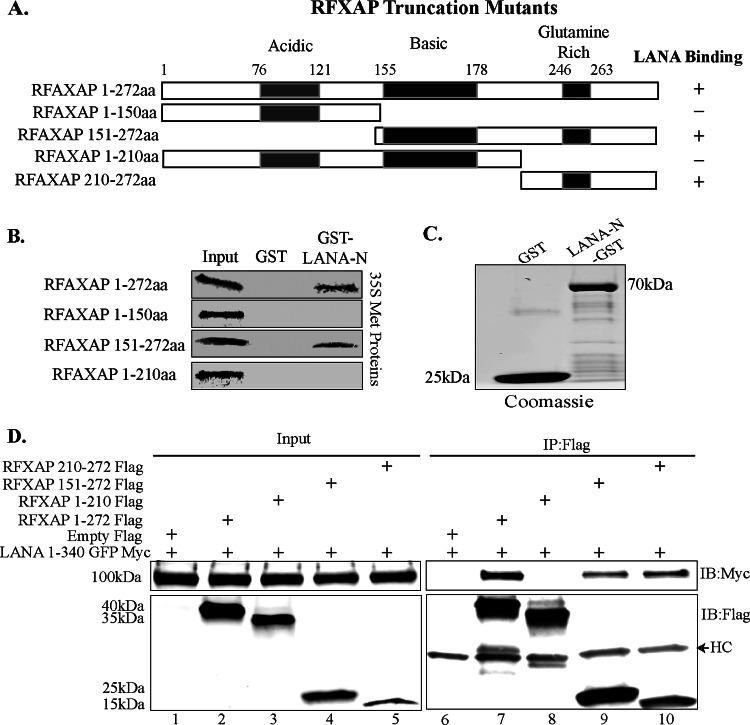
FIG 4.
The C terminus of RFXAP interacts with LANA. (A) Schematic showing RFXAP domains used for coimmunoprecipitation and GST pulldown assays. (B) In vitro-translated full-length RFXAP (aa 1 to 172) or the truncated RFXAP mutant spanning aa 1 to 150, aa 151 to 272, or aa 1 to 210 was pulled down with GST or LANA-N–GST and resolved on SDS-PAGE gels, followed by detection by autoradiography. (C) Expression of control GST and LANA-N–GST proteins that were used for GST pulldown of RFXAP truncation mutants. (D) Coimmunoprecipitation assay of the N terminus of LANA and different truncation mutants of RFXAP. HEK293T cells were transiently cotransfected with LANA-N–GFP–Myc and either the empty control vector pA3F, full-length Flag-tagged RFXAP (aa 1 to 272) or the truncated RFXAP protein spanning aa 1 to 210, aa 150 to 272, or aa 210 to 272. The cell lysates were immunoprecipitated with anti-Flag antibody, and immunoprecipitated proteins were immunoblotted with anti-Flag (mouse) and anti-Myc antibodies. HC represents the heavy chain detected because mouse anti-Flag was used for the detection of Flag-tagged proteins.