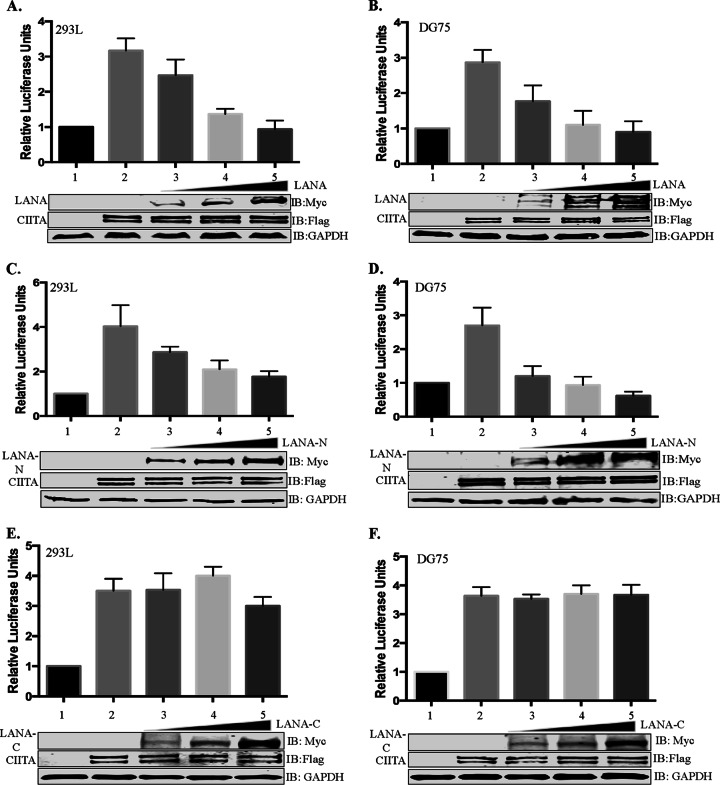
FIG 6.
LANA inhibits transcriptional activity of the HLA-DRA promoter. Luciferase reporter assays show a dose-dependent reduction of the luciferase activity mediated by the HLA-DRA promoter when CIITA-expressing cells were cotransfected with full-length LANA or the amino-terminal region of LANA but not with the carboxy-terminal region of LANA. For the reporter assay, the HLA-DRA promoter fused with the luciferase reporter was transiently transfected into HEK293L cells (A, C, and E) or DG75 cells (B, D, and F). The cells were cotransfected with CIITA to stimulate the HLA-DRA promoter along with either full-length LANA (A and B), the RFXAP-interacting domain of LANA, the amino-terminal domain of LANA (C and D), or the carboxy-terminal domain of LANA (LANA-C) (E and F). The cell lysates were used for measuring luciferase activities using a dual-luciferase assay kit. Relative luciferase units were calculated relative to the basal promoter activity. Lane 1, basal DRA-luciferase activity; lane 2, CIITA-stimulated DRA-luciferase activity; lane 3, CIITA-stimulated DRA-luciferase activity with 1 μg of LANA or a truncation (LANA-N or LANA-C); lane 4, CIITA-stimulated DRA-luciferase activity with 2 μg of LANA or a truncation (LANA-N or LANA-C); lane 5, CIITA-stimulated DRA-luciferase activity with 3 μg of LANA or a truncation (LANA-N or LANA-C). The error bars represent standard deviations of the means from at least three experimental replicates. Equal amounts of cell lysates from each experimental set were used for the detection of overexpressed CIITA and LANA proteins. The GAPDH immunoblot shows equal loading of the cell lysates.