Abstract
Background
Coping strategy impacts susceptibility to psychosocial stress. The locus coeruleus (LC) and dorsal raphe (DR) are monoamine nuclei that are implicated in stress-related disorders. This study was designed to identify genes in these nuclei that distinguish active and passive coping strategies in response to social stress.
Methods
Rats were exposed to repeated resident-intruder stress and coping strategy determined. Gene and protein expression in the LC and DR were determined by PCR array, ELISA, and compared between active and passive stress coping and unstressed rats. The effect of daily IL-1 receptor antagonist (IL-1ra, ICV) prior to stress on anhedonia was also determined.
Results
Rats exhibited passive or active coping strategies based on a short (SL) or longer latency (LL) to assume a defeat posture, respectively. Stress differentially regulated 19 and 26 genes in the LC and DR of SL and LL rats, respectively, many of which encoded for inflammatory factors. Notably, IL1β was increased in SL and decreased in LL rats in both the LC and DR. Protein changes were generally consistent with a proinflammatory response to stress in SL rats selectively. Stress produced anhedonia selectively in SL rats and this was prevented by IL-1ra, consistent with a role for IL1β in stress vulnerability.
Conclusions
This study highlighted distinctions in gene expression related to coping strategy in response to social stress. Passive coping was associated with a bias towards pro-inflammatory processes, particularly IL1β, whereas active coping and resistance to stress-related pathology was associated with suppression of inflammatory processes.
Keywords: Social defeat, affective disorders, coping, inflammation, Interleukin 1β, susceptibility
INTRODUCTION
Stressors of a social nature are a common form of stress for humans, including abuse and bullying (1). The inability to successfully adapt to stress produces pathological changes that can lead to psychological disorders such as depression and anxiety and comorbid medical disorders including diabetes, irritable bowel syndrome and cardiovascular disease (2–7). Interestingly, striking individual differences arise in the pathogenic potential of a stressor, rending one more or less likely to develop stress-related pathologies. Evidence suggests that individual differences in stress-induced pathology are related to coping style. For example, a submissive personality characterized by passive coping has been associated with vulnerability to psychopathology (8, 9), irritable bowel syndrome (10), and hypertension (11–13) while active coping has been related to increased resiliency (14). Therefore, identifying the biology underlying different coping mechanisms may reveal systems or substrates that determine resilience or vulnerability to stress-related pathologies.
Social stress has been modeled in rodents through the use of the resident-intruder paradigm (15). We previously reported that, like humans, robust individual differences in the coping response to social stress emerge in an outbred population of Sprague Dawley rats, resulting in two phenotypes (16, 17). One phenotype exhibits passive coping behaviors characterized by the assumption of a supine defeat posture within a short latency (termed SL). The SL phenotype develops stress-induced behavioral, neuroendocrine, and cardiovascular changes similar to those occurring during depression (16, 18). In contrast, the alternate phenotype adopts a proactive coping strategy resulting in increased defeat latencies (LL rats) and more upright postures and displays a general resistance to many of the pathological consequences observed in the SL phenotype (16–18). Phenotypic differences in coping mechanisms appear to have a strong genetic link because artificial selection for either extreme results in distinct genotypes within only a few generations (19). Furthermore, it has long been recognized that genetic differences in humans may affect vulnerability to psychiatric disorders, in part by influencing coping behaviors (20). Our previous work has highlighted numerous peripheral and central social stress-induced adaptations that occur in only a subpopulation of the same rat strain, suggesting that genetic variability within the outbred population may be driving these differences (16, 17). The stress-sensitive brain regions, the locus coeruleus (LC) and dorsal raphe nucleus (DR) are source nuclei of the major brain monoamines that play a role in the behavioral stress coping response (21–25). Importantly, these two monoaminergic systems are also implicated in the pathophysiology of depression and therefore represent candidate brain regions that link stress to psychopathology. The unique passive stress coping style of the Wistar Kyoto rat (WKY) was previously shown to exhibit differential gene expression in the LC and DR (26). More recently, we reported opposing stress-induced neuroadaptations in the corticotropin-releasing factor system (CRF) within the DR of SL versus LL rats (17). Therefore, the present study compared the effects of stress on gene expression within the LC and DR of SL, LL, and control rats. Notably, due to opposing adaptations observed in genes encoding for inflammatory factors within the brain, these studies further identified changes in circulating and brain levels of inflammatory proteins and tested a causal role for IL1β in a pathological consequence of stress.
METHODS
Animals
Male Sprague Dawley rats (225–250g; intruder or controls) and Long-Evans retired breeders (650–850g; residents, Charles River, Wilmington MA) were individually housed in standard cages with free access to food and water on a 12-hr light/dark cycle. The Institutional Animal Care and Use Committee (IACUC) at the Children’s Hospital of Philadelphia and the University of South Carolina approved these studies and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Social stress (resident-intruder) model
The social defeat procedure used was a modified version of the resident-intruder paradigm originally developed by Miczek (15) and identical to that used in our previous studies (16–18). Briefly, rats were randomly assigned to the “intruder” or “control” group. Intruders were exposed to a novel Long-Evans retired breeder for 30-mins on five consecutive days. All Long-Evans rats were prescreened for their level of aggression and were only used in this study if they attacked within 60 sec. The control group was exposed to a novel cage for 30-mins daily. Average defeat latencies (latency to exhibit a supine submissive posture) were recorded. Intruder and control rats used in the gene expression studies were euthanized 24h after the 5th control or defeat exposure. Separate cohorts of rats were euthanized either 1 or 24h after the final social stress or control exposure for protein analysis. For the IL-1ra treatment study, a separate cohort of rats was euthanized 11 days after the final social defeat or control.
Tissue collection
Brains were sliced coronally until caudal LC (Bregma −10.08, Paxinos and Watson) and DR (Bregma −8.04). For PCR analysis LC sections were mounted onto RNAse-free slides and LC tissue was micro-chiseled using an Eppendorf MicroDissector. DR tissue was collected using a 2mm punch at the midline just below the aqueduct using a 1mm wide trephine. LC and DR tissue was stored in RNALater (Qiagen) at −80°C. For protein analysis the LC and DR were micro-dissected using a 1mm wide trephine at 1mm or 2mm depth, respectively and stored at −80°C.
RNA isolation & PCR array
Total RNA was isolated using Qiagen® RNeasy Micro kit as per the manufacturer’s instructions. The quality and concentration of final RNA yield was determined by using a ND-1000 NanoDrop UV spectrophotometer (ThermoFisher, Wilmington, DE) and 2100 Bioanalyzer RIN (Agilent Technologies, Santa Clara, CA). RNA was converted to cDNA using the RT2 First Strand Kit (SABiosciences, Valencia, CA). Samples were run on a quantitative RT2 Profiler PCR array (SABiosciences) containing 88 genes involved in G-protein coupled receptor signaling (GPCR Signaling PathwayFinder™) and run on a real-time PCR machine (SDS-7500, Applied Biosystems). Data were quantified using the comparative cycle threshold (Ct) method and normalized to the housekeeping genes Hprt1 and Ldha.
Ingenuity Pathway Analysis
Fold change data were analyzed using the Ingenuity Pathway Analysis software (IPA, Ingenuity Systems) to identify biological networks of genes that were altered greater than 1.5 fold in socially stressed rats compared to controls. A Fisher’s exact test (p value <0.05) was calculated to validate the association of a biological disease.
Determination of cytokine levels
LC and DR tissue were homogenized in RIPA buffer (Sigma, St Louis, MO) with Protease and Phosphatase Inhibitor (Pierce, Rockford, IL). Homogenized brain tissue and plasma were assayed for IL1β (R&D Systems, Minneapolis, MN), MCP-1 and IL-10 (Invitrogen, Camarillo, CA), IL-6 (Pierce Biotechnology, Rockford, IL) using an ELISA according to each manufacturer’s protocol. Each protein of interest was expressed as a ratio to total protein.
Intracerebroventricular IL-1ra Treatment
A permanent guide cannula (22 ga., Plastics One, Roanoke, VA) was implanted into the right lateral ventricle (AP, −0.8 mm relative to bregma; ML, −1.5 mm; DV, −4.2 mm from the skull). Rats were allowed 10 days to recover at which time the innate immune response to the cannula is absent or substantially diminished (Whittle et al., 1998). Histological verification of cannula placement was verified by injecting 5µL of 1% Chicago Sky Blue.
Rats were microinjected with 2.5 µL (0.25 µg/rat ICV, infused over 3 min) of recombinant rat IL-1 receptor antagonist (IL-1ra, R & D Systems) or vehicle (0.1% BSA in sterile PBS) 30 min prior to daily social defeat. This dose of IL-1ra blocks lipopolysaccharide-induced hyperthermia (27) and impairments in fear conditioning (28).
Sucrose Preference
Rats had free access to food, water and 1% sucrose solution for 48h prior to testing. At 1200 on the test day (2–3 days prior to stress/control exposure (baseline measurement) and 10 days after the final social defeat or control exposure), both bottles were removed. Bottles were returned at 1900 and total weight of liquid drank from each bottle between 1900 and 2200 was calculated. Sucrose preference was determined by dividing the weight of 1% sucrose consumed by the total weight of all liquid consumed.
Data analysis
To define the subpopulations of rats, separate K-means cluster analyses (JMP 9.0; SAS, Cary, NC) were conducted on average defeat latencies. One-way ANOVAs followed by Tukey’s post-hoc analyses were used to determine statistical significance of cytokine protein levels between control, SL and LL rat’s samples. P-values <0.05 were considered significant.
RESULTS
Individual differences in the coping response to social stress
Consistent with our previous studies (16, 17), the average defeat latency of the outbred population of Sprague Dawley rats was bimodally distributed. Of the 46 rats used for PCR analysis, the SL cluster had latencies ranging from 95–387s (272±22, n=28) and the LL cluster exhibited latencies to defeat between 423–876s (599±23, n=18; p<0.0001). In a separate cohort, 40 rats were exposed to social stress and used for protein analysis; the SL cluster consisted of defeat latencies ranging from 81–282s (Kmeans ±SEM: 191±14s, n=27) and the LL cluster exhibited defeat latencies between 441–740s (482±28s, n=13; p<0.0001).
Social stress-induced changes in gene expression in the LC and DR
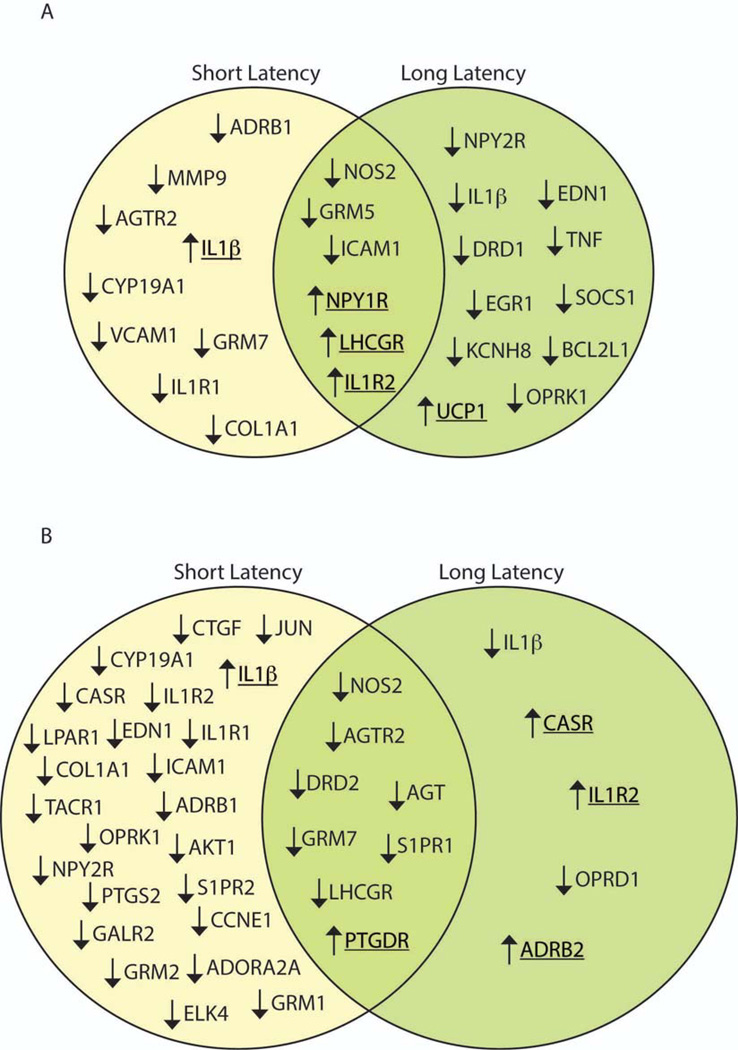
Comparative threshold analysis revealed a total of 15 genes (11 down-, 4 up-regulated) in the LC of SL rats and 17 genes (13 down, 4 up) in LL rats that were differentially expressed by 1.5 fold or greater relative to controls (Table S1). Figure 1 and Table 1 provide a direct comparison of genes impacted by social stress in SL versus LL rats. In the LC, 6 genes were similarly regulated by stress in SL and LL phenotypes (3 down, 3 up) (Fig. 1A). Shared downregulated genes included NOS2 and GRM5, while those upregulated by stress in all defeated rats included the neuropeptide Y receptor 1 (NPY1R) and the interleukin receptor IL1R2 (Fig. 1A). Notably, stress had opposing effects on IL1β, increasing expression in the LC of SL rats and decreasing expression in LL rats (Table. 1). Furthermore, several genes differentially expressed were unique to either SL or LL rats (Fig. 1A, Table. 1).
Figure 1.
Venn diagrams of genes differentially expressed in the LC (A) and DR (B) of short latency (left circle) and long latency (right circle) rats following social defeat. Arrows signify the relative change in gene expression compared to controls with underlined genes indicating an increase in expression. In both the LC and DR less than one-quarter of the genes altered by social stress were shared between SL and LL rats, indicated by the overlap in the middle of the Venn diagram.
Table 1.
Comparative analysis of gene expression profiles relative to controls within the LC
| Genes with the same directional change | |||
| Long latency | Short latency | ||
| NOS2 | ↓−3.932 | ↓−3.355 | |
| GRM5 | ↓−2.727 | ↓−3.345 | |
| ICAM1 | ↓−2.571 | ↓−1.995 | |
| IL1R2 | ↑4.310 | ↑7.976 | |
| NPY1R | ↑1.745 | ↑2.198 | |
| LHCGR | ↑1.550 | ↑2.418 | |
| Genes with the opposing directional change | |||
| Long latency | Short latency | ||
| IL1β | ↓−3.199 | ↑3.993 | |
| Unique genes | |||
| Long latency | Short latency | ||
| NPY2R | ↓−3.664 | ADRB1 | ↓−4.759 |
| EDN1 | ↓−2.794 | AGTR2 | ↓−3.016 |
| DRD1 | ↓−2.321 | CYP19A1 | ↓−2.335 |
| TNF | ↓−2.089 | MMP9 | ↓−2.326 |
| EGR1 | ↓−2.028 | VCAM1 | ↓−2.208 |
| SOCS1 | ↓−1.815 | GRM7 | ↓−2.068 |
| KCNH8 | ↓−1.686 | COL1A1 | ↓−1.786 |
| BCL2L1 | ↓−1.681 | IL1R1 | ↓−1.636 |
| OPRK1 | ↓−1.533 | ||
| UCP1 | ↑1.615 | ||
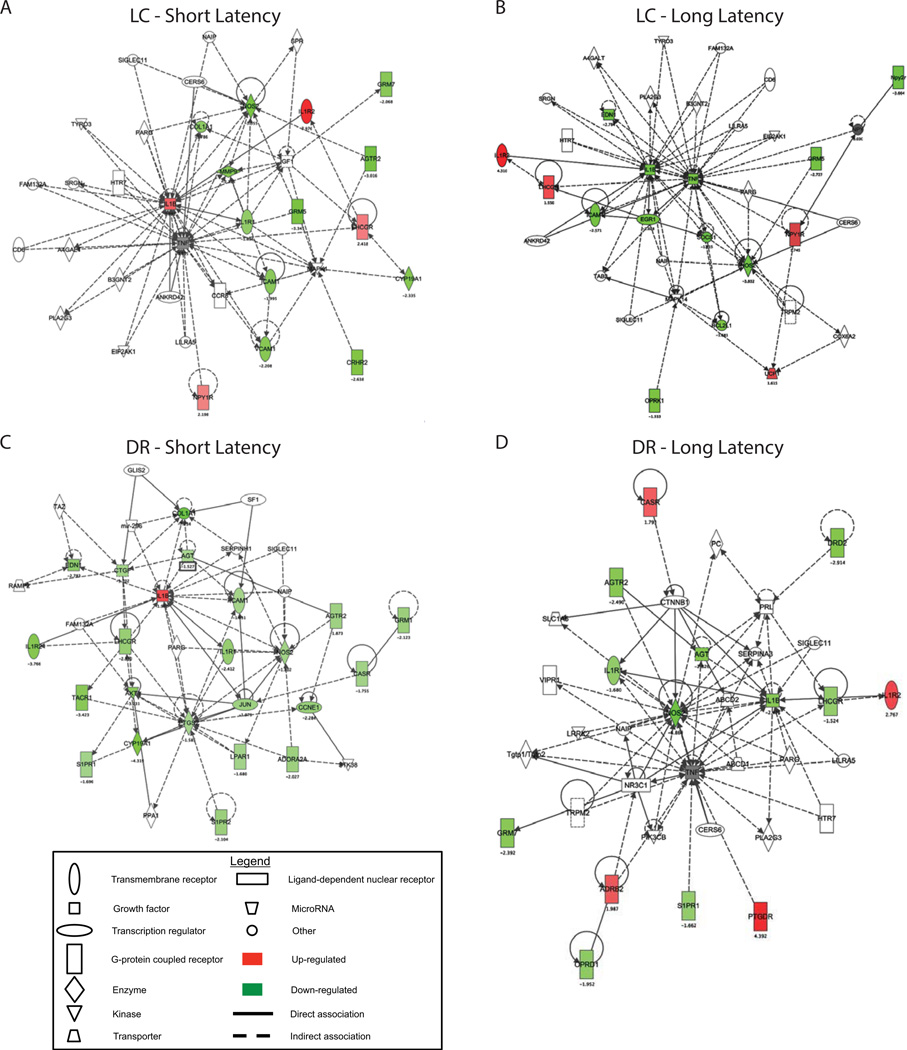
IPA analysis identified gene networks that are differentially expressed following social stress. Interestingly, for both phenotypes the most significantly affected functional gene networks regulated by stress in the LC were networks of which IL1β and NOS2 were major hubs. Additionally, TNF-α, which was downregulated in LL rats, was a major hub in both pathways (Fig. 2A,B). For both SL and LL rats 15 of 35 genes in the networks were stress-regulated.
Figure 2.
Ingenuity-based interactome analysis of differentially expressed genes. Following social defeat stress, IPA identified the most significantly altered functional networks in the LC (A and B) and in the DR (C and D) based on defeat latency. A network score of >2 indicates a ≥99% confidence that the genes in the network are not associated by chance. Network scores are as follows: LC, short latency: 37 (16 differentially regulated genes); LC, long latency: 36 (16 differentially regulated genes); DR, short latency: 53 (23 differentially regulated genes); DR, long latency: 35 (15 differentially regulated genes). Each node is depicted by a symbol that represents the functional class of the gene. The symbols without color are those selected by IPA to produce networks.
Social stress also regulated gene expression within the DR and SL rats were particularly affected with nearly three times the number of genes that were differentially expressed (>1.5 fold) versus controls as compared with LL rats (Table S2). For SL rats 32 genes were regulated (30 down- and 2 up-regulated). For LL rats 13 genes were regulated (9 down-and 4 up-regulated). Notably, IL1βωασονεοϕ only 2 genes that were upregulated in SL rats. Stress had similar effects on the expression of 8 genes in the DR of SL and LL rats (Fig. 1B, Table 2). Consistent with findings in the LC, certain genes encoding for proteins involved in immune function were changed in opposing directions; IL1β was suppressed in LL and increased in SL rats and the IL1R2 gene was upregulated in the DR of LL rats and suppressed in SL rats. Furthermore, several gene changes were unique to either SL or LL rats. Another striking difference between phenotypes was the abundance of metabotropic glutamate receptors suppressed in SL rats (GRM1, 2 and 7) while only GRM7 was downregulated in LL rats (Fig. 1B, Table 2).
Table 2.
Comparative analysis of gene expression profiles relative to controls within the DR
| Genes with the same directional change | |||
| Long latency | Short latency | ||
| NOS2 | ↓−6.868 | ↓−1.802 | |
| DRD2 | ↓−2.914 | ↓−1.500 | |
| AGT | ↓−2.826 | ↓−1.527 | |
| AGTR2 | ↓−2.490 | ↓−1.873 | |
| GRM7 | ↓−2.392 | ↓−1.929 | |
| S1PR1 | ↓−1.662 | ↓−1.696 | |
| LHCGR | ↓−1.524 | ↓−2.310 | |
| PTGDR | ↑4.392 | ↑2.707 | |
| Genes with the opposing directional change | |||
| Long latency | Short latency | ||
| IL1β | ↓−2.475 | ↑1.932 | |
| CASR | ↑1.793 | ↓−1.755 | |
| IL1R2 | ↑2.767 | ↓−3.766 | |
| Unique genes | |||
| Short latency | Short latency | ||
| COL1A1 | ↓−7.234 | ADORA2A | ↓−2.027 |
| CYP19A1 | ↓−4.319 | JUN | ↓−1.975 |
| TACR1 | ↓−3.423 | ADRB1 | ↓−1.724 |
| AKT1 | ↓−3.231 | GRM2 | ↓−1.715 |
| EDN1 | ↓−2.783 | CTGF | ↓−1.702 |
| NPY2R | ↓−2.616 | LPAR1 | ↓−1.680 |
| IL1R1 | ↓−2.412 | ICAM1 | ↓−1.631 |
| CCNE1 | ↓−2.284 | OPRK1 | ↓−1.623 |
| GALR2 | ↓−2.142 | PTGS2 | ↓−1.585 |
| GRM1 | ↓−2.123 | ELK4 | ↓−1.575 |
| S1PR2 | ↓−2.104 | ||
| Long latency | |||
| OPRD1 | ↓−1.952 | ||
| ADRB2 | ↑1.987 | ||
Similar to the LC, for both phenotypes genes that were significantly regulated by stress in the DR were components of functional networks of which IL1β and NOS2 were major hubs (Fig. 2C, D). For SL rats 23 of the 35 genes in the network were stress-regulated and for LL rats, 14 of 35 genes were stress-regulated.
Cytokine protein levels in SL and LL rats
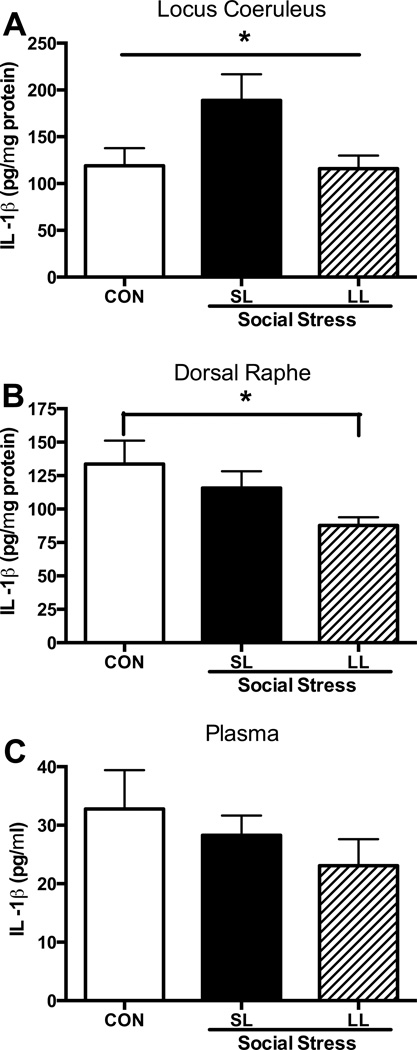
The effects of social stress on protein levels of inflammatory markers were investigated and found to be consistent with some of the stress-induced changes in IL1β gene expression. IL1β protein levels were significantly increased in the LC of SL rats above that of controls (F(2,16)=3.7, p<0.05) and of LL rats 24-hrs after the last stress (p<0.05, Fig. 3A). At the same time, IL1β protein levels were significantly decreased in the DR of the LL phenotype compared with controls (F(2,17)=3.4, p=0.05; Fig. 3B). No changes were detected in plasma at this time (Fig. 3C). In a separate cohort of rats, 10 days after the final exposure to stress or control, SL rats maintained a modest, yet significant increase in IL1β levels in the LC as evidenced by IL1β (pg/µg total protein) levels nearly 120% of control levels (CON: 0.27±0.01, SL: 0.33±0.01; t(10)=1.9; p=0.044) while LL rats were comparable to control (LL: 0.28±0.03; p=0.37). Furthermore, at this same time there was a strong trend for IL1β levels to be negatively correlated with defeat latency (r= −0.53, p=0.052). There was no effect of stress on the acute inflammatory response in plasma determined 1 hr after the 5th stress/control exposure (p=0.25), DR (p=0.56) or LC (p=0.34) (Data not shown).
Figure 3.
IL-1β protein levels 24-hrs after the 5th social stress or control exposure. While there were no significant effects of stress on IL-1β 1-hr after the 5th exposure, 24-hrs later (A) social stress increased IL-1β protein levels selectively in the LC of short latency rats. (B) Alternatively, IL-1β in the DR of LL rats was significantly suppressed compared with controls. (C) There were no effects of social stress on plasma IL-1β levels. *p<0.05, Tukey’s post-hoc. Average defeat latencies for rats included in the 24-hr post-stress group were 173±18 for SL and 491±56 for LL and were significantly different (t(21)=7.1; p<0.0001).
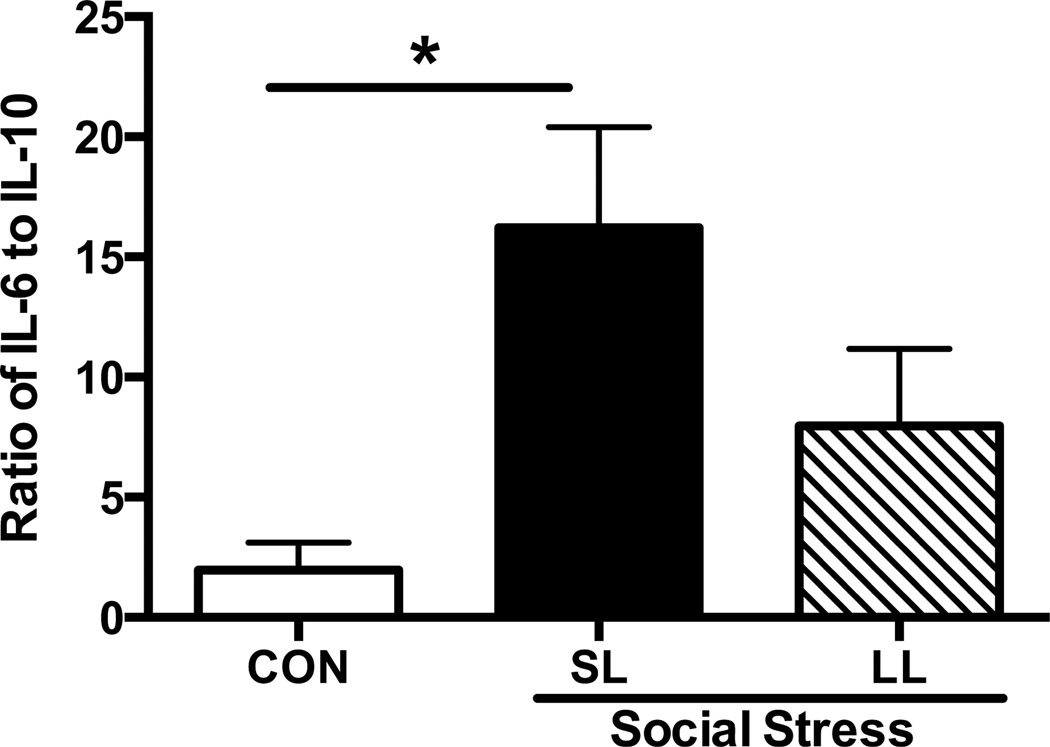
The balance between circulating pro-inflammatory (IL-6) and anti-inflammatory (IL-10) cytokines was assessed in the plasma 1-hr and 24-hrs after the 5th and final manipulation (Fig. 4). The ratio of IL-6 to IL-10 was significantly higher in the SL rats 1-hr after social stress (F(2,10)=6.2; p=0.018) but not 24-hrs later (F(2,12)=0.45; p=0.65; Con: 23.7±9, SL: 37±9, LL: 31±12).
Figure 4.
Ratio of pro- (IL-6) to anti-inflammatory (IL-10) cytokines in the plasma of control and socially stressed rats. There was a significant effect of stress observed 1-hr after the 5th exposure to social stress; SL rats exhibited a significant stress-induced increase in the IL-6 to IL-10 ratio compared with controls. Social stress did not have an enduring effect on the IL-6 to IL-10 ratio, as there were no significant differences between control, SL, or LL rats 24-hrs after the 5th exposure to social stress (data not shown, p=0.65). Average defeat latencies for rats included in the 1-hr post-stress group were 209±23 for SL and 455±27 for LL and were significantly different (t(13)=6.5; p<0.0001).
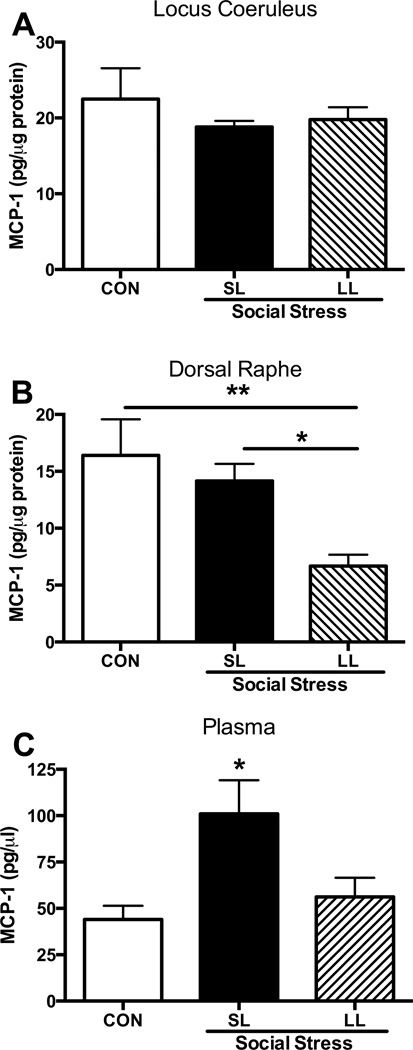
Social stress had unremarkable effects on monocyte recruitment as measured by MCP-1 levels 1-hr after the 5th social stress within the plasma (p=0.29), DR (p=0.2) and the LC (p=0.4) (data not shown). However, by 24-hrs after the 5th exposure MCP-1 plasma levels were significantly elevated in SL rats (F(2,25)=3.5; p<0.05), whereas levels in LL rats were comparable to controls (Fig. 5C). In the DR MCP-1 was suppressed in LL rats compared with control and SL rats (F(2,16)=6.7; p=0.008, Fig. 5B). There were no stress-induced effects on MCP-1 levels within the LC (p=0.4, Fig. 5A).
Figure 5.
MCP-1 protein levels 24-hrs after the 5th social stress or control exposure. (A) Social stress had no effect on MCP-1 protein levels in the LC. (B) Within the DR, LL rats exhibited a social stress-induced suppression of MCP-1. (C) Social stress produced an increase in MCP-1 levels in the plasma of SL rats compared with controls. *p<0.05, Tukey’s post-hoc vs. control.
Effects of recombinant IL-1ra treatment on coping strategy and stress-induced anhedonia
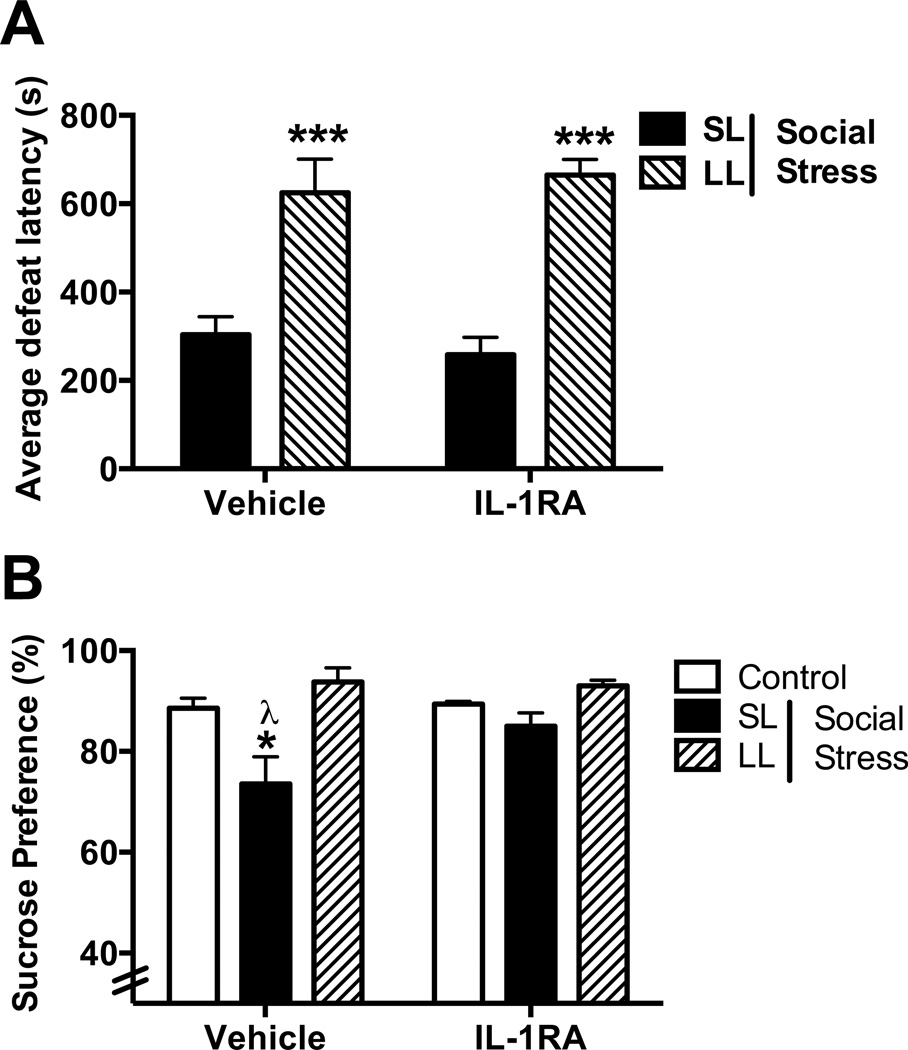
K-means cluster analysis of rats treated with vehicle or IL-1ra were analyzed separately. Vehicle-treated rats in the SL and LL cluster exhibited latencies between 131–435s (303±40s) and 515–771s (625±75), respectively. IL-1ra-treated rats in the SL and LL cluster exhibited latencies between 83–345s (258±40) and 622–735s (665±35s), respectively. The average defeat latencies exhibited by rats in the SL and LL clusters were significantly different (Fig. 6, F(1, 16)=48.78; p<0.0001), with active coping LL rats exhibiting significantly greater defeat latencies than passive coping SL rats in both vehicle- (p=0.001) and IL-1ra-treated groups (p=0.001) demonstrating no effect of treatment on defeat latency (p=0.96). A similar percentage (27%) of vehicle-treated rats and IL-1ra-treated rats (33%) were classified as LL, confirming that IL-1ra treatment had no effect on coping strategy.
Figure 6.
Effect of IL1ra treatment on defeat latency and social stress-induced anhedonia. (A) Active coping LL rats exhibit significantly greater defeat latencies than passive coping SL rats in both the vehicle- and IL-1ra-treated groups (***p<0.001 vs. SL). IL-1ra had no effect on coping strategy. (B) Vehicle-treated passive coping SL rats display anhedonia as measured by decreased preference for 1% sucrose over water as compared with vehicle-treated control (*p<0.05) and LL rats (λp<0.05). SL rats that were treated with IL1ra (0.25 µg/rat, icv) prior to each social defeat episode did not develop anhedonia (p>0.05, Tukey post-hoc vs. control).
Sucrose preference in rats randomly assigned to the control/stress × treatment group did not differ prior to stress exposure (average sucrose preference for the 6 different groups ranged from 86.6% to 89.5% (F(5,21) = 0.13; p=0.4) (Data not shown). Repeated social stress produced anhedonia as indicated by a decrease in sucrose preference in vehicle-treated SL rats, compared to both LL and control rats (Fig. 6; F(2,22)=6.8; p=0.005, Tukey post hoc p<0.05). However, anhedonia was prevented in SL rats treated with recombinant IL-1ra as indicated by a similar preference for 1% sucrose over water compared with controls (Fig. 6; p>0.05).
DISCUSSION
Previous studies have compared gene expression patterns in brains of different strains of rats known to exhibit differing coping styles (26, 29). The present study is novel in being the first to report regulation by social stress of distinct sets of genes within rats of the same strain exhibiting divergent coping strategies. A preponderance of genes related to inflammatory processes were regulated by social stress for both phenotypes, highlighting the dynamics of inflammatory processes in brain in response to stress. Notably, the passive SL phenotype was biased towards pro-inflammatory processes whereas the active LL phenotype exhibited adaptations buffering these processes. Evidence of opposing regulation of IL1β in SL and LL rats and the finding that SL rats develop anhedonia that is sensitive to IL1β antagonists implicate IL1β as one determinant in vulnerability related to coping style. As we previously reported the SL phenotype selectively demonstrates several behavioral and neuroendocrine features comparable to depressed patients, while the LL phenotype is resistant to these changes (16), IL1β and other differentially regulated genes identified in the present study are potential biomarkers of stress susceptibility or resilience.
Stress-induced changes in the LC
Highlighting stress-induced changes within the LC is relevant given the role of the LC in stress coping (21) and stress-related psychiatric disorders (30, 31), and may reveal critical genes related to stress susceptibility. An analogous gene expression analysis within human LC revealed alterations in multiple signaling pathways that were distinct in depressed patients (32). In the present study, less than one-third of the LC genes that were differentially expressed in defeated rats were similarly altered in both phenotypes.
One striking difference between the phenotypes was the opposing effects of stress on cytokine-related gene expression. In the passive coping phenotype, both the gene and protein encoding the pro-inflammatory cytokine, IL1β, were elevated in LC. In the active coping phenotype the genes encoding for the pro-inflammatory cytokines IL1β and TNF-α were decreased. IL1β in LC has been shown to increase neuronal activity (33) and studies evaluating the effects of an IL-1 receptor antagonist suggest that tonic LC excitation may be regulated in part by IL1β (33). Importantly, antidepressants have been reported to attenuate inflammation-induced brain cytokine production, decrease activity of LC neurons and reduce depressive-like symptoms, further supporting a role for proinflammatory factors in depressive disorders (34–36). Future studies determining the impact of IL-1 blockade on LC neuronal activity of socially stressed rats may reveal this as one mechanism by which IL-1ra promotes stress resilience (ie. antidepressant-like activity).
Considerable evidence also points towards the central NPY system as protecting against anxiety- and depressive-like responses (37–39). Specifically, stimulation of NPY1 and inhibition of NPY2 receptors produce prominent anxiolytic and antidepressant effects (38, 40, 41). NPY is co-localized with norepinephrine in LC neurons and is inhibitory to LC neuronal firing (42, 43). The finding that social stress upregulated NPY1R gene expression in the LC of both phenotypes suggests that this may be an adaptive stress response in both groups while suppression of the NPY2R gene in active coping rats could have additive effects promoting resilience. The suppression of the gene encoding the kappa opioid receptor 1 in the LC of active coping rats may also confer resilience. The dynorphin-kappa opioid receptor system has been implicated in the aversive effects of stress (44, 45) illustrated by its upregulation in the LC of the stress-sensitive WKY rat, a strain exhibiting passive coping style. Furthermore, kappa opiate receptor antagonists have selective antidepressant potential in this strain (26, 46–48).
Stress-induced changes in the DR
In addition to the LC-NE system, the DR-serotonin (5-HT) system mediates behavioral aspects of the stress response and is implicated in the pathophysiology of depression (49, 50). The DR of passive coping (SL) rats was particularly sensitive to social stress as indicated by the higher number of genes affected compared to all other groups. Similar to effects observed in LC, social stress targeted genes related to inflammation in the DR, producing opposing effects in passive and active coping rats. In SL rats a decrease in expression of the gene encoding for the IL1R1 receptor occurred most likely as an adaptive response to excess IL1β. Interestingly, the gene encoding for the IL1R2 “decoy” receptor, an endogenous inhibitor of IL-1, was increased in LL and decreased in SL rats, revealing one adaptation evident in active coping rats that may dampen the inflammatory response within the DR (51). In LL rats, elevated IL1R2 findings are accompanied by suppressed IL1β gene and protein expression and decreased MCP-1 protein. Importantly, inflammatory cytokines have the potential to regulate the activity of the DR-5-HT system. Evidence suggests that IL1β may inhibit serotonergic cell firing and increase indoleamine 2–3-dioxygenase (IDO), one of the most robust biomarkers for depression, thereby leading to increased tryptophan metabolism and lower levels of 5-HT (52, 53). As a result, the stress-induced decrease in cytokines evident in the LL phenotype may promote resilience by maintaining or increasing 5-HT levels in the presence of stress. Together, these data support the notion that proactive stress coping may protect against the pathogenesis of depression through suppression of pro-inflammatory responses. Studies designed to reveal the interaction between IL1β and 5-HT within the DR of active coping rats will advance our understanding of putative mechanisms promoting resilience to psychiatric disorders.
Critical role of central IL1β in the depressive-like phenotype in passive coping rats
Previously, we demonstrated that following social stress SL rats exhibit endocrine and behavioral endpoints of depression in rodent models, including increased immobility in the forced swim test that can be blocked by prior antidepressant administration (16, 18), endocrine endpoints including exaggerated HPA axis activation and adrenal hypertrophy (16), and decreased heart rate variability (18). The current study added another endpoint of depressive-like behavior, anhedonia, to those that are selectively expressed by SL rats. Elevated neuroinflammation may play a central role in depressive and anxiety-like behaviors. Elevated levels of IL1β in the CSF of depressed patients are correlated with the duration and severity of symptoms (54). In addition, IL1β administration induces anhedonia, anorexia, and impaired social interaction, all key symptoms observed in depressed patients (55–59). The endogenous protein IL1ra, binds to the IL-1 receptor but does not induce a biological response, thereby acting as an IL1β antagonist (57, 60, 61). Taken with the finding that IL1β is increased selectively in the SL rat, the ability of the endogenous antagonist of the IL-1 receptor, IL1ra, to attenuate social stress-induced anhedonia supports a causal role for this cytokine in this endpoint of depression. Notably, the IL1 antagonist did not affect coping style indicating that IL1β is not a determinant of coping style. Rather, increases in IL1β that occur selectively in the SL rat contribute to anhedonia and are downstream from factors that determine coping style. The results imply that IL1β plays a role in promoting susceptibility to the depressive-like phenotype following social defeat stress in passive coping individuals.
Stress-induced changes in circulating inflammatory-related proteins
In addition to neuroinflammation, circulating cytokine levels are also implicated in stress-related disorders (62–64). Importantly, Infliximab, a monoclonal antibody against TNF-α, exhibited antidepressant efficacy in a subset of patients characterized by elevated plasma cytokines (65). In the present study there was a striking increase in the ratio of pro- to anti-inflammatory cytokines selectively in the passive coping SL phenotype in response to social stress. Chemoattractant cytokines also play an important role during inflammation, directing the migration of inflammatory cells such as monocytes and T-lymphocytes. MCP-1 is in part responsible for monocyte recruitment (66) and contributes to inflammatory-related disorders such as arthritis and atherosclerosis (67). Although the role of MCP-1 in major depression is equivocal, disturbances within the MCP-1 system are associated with major depression (68, 69). Taken together, these studies are consistent with the concept that pathology associated with passive stress coping is related in part to a hyperresponsive immune system.
SUMMARY
Studies demonstrating that stress management/coping therapy has therapeutic efficacy in depression have established an association between coping strategies and susceptibility to stress-related pathology (70–73). Inefficient adaptation to stress results in psychiatric and medical disorders such as depression, hypertension and irritable bowel syndrome (7, 74). The complex interactions between systems such as the immune, neuroendocrine and autonomic nervous systems suggest that a dynamic interaction between multiple factors contribute to the pathogenesis of stress-related disorders.
The current study is the first to relate coping style (or strategy) during social stress, a factor known to determine stress consequences, to changes in gene expression in monoamine nuclei. This study highlights the involvement of inflammation in susceptibility to social stress-induced pathologies and revealed several neurobiological adaptations associated with diverse coping strategies, uncovering potential biomarkers that impact stress susceptibility. Future studies targeting these neurobiological substrates will be important in determining how these genes impact behavior and susceptibility to stress-related diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Eric Rappaport and the staff of the Nucleic Acid and protein core at the Children’s Hospital of Philadelphia for technical support. The authors would also like to thank Anna Capps for her technical assistance. This work was supported by National Institute of Health Grants MH058250, MH040008 and 5P20GM103641, the Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator Award No. 17830 to SKW and the American Heart Association #13BGIA14370026.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science. 2005;14:64–68. [Google Scholar]
- 2.Albus C. Psychological and social factors in coronary heart disease. Ann Med. 2010;42:487–494. doi: 10.3109/07853890.2010.515605. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 4.Friedmann E, Thomas SA, Liu F, Morton PG, Chapa D, Gottlieb SS. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152:940 e941–948 e941. doi: 10.1016/j.ahj.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Campayo A, Gomez-Biel CH, Lobo A. Diabetes and depression. Curr Psychiatry Rep. 2011;13:26–30. doi: 10.1007/s11920-010-0165-z. [DOI] [PubMed] [Google Scholar]
- 6.Fenton WS, Stover ES. Mood disorders: cardiovascular and diabetes comorbidity. Curr Opin Psychiatry. 2006;19:421–427. doi: 10.1097/01.yco.0000228765.33356.9f. [DOI] [PubMed] [Google Scholar]
- 7.Folks DG. The interface of psychiatry and irritable bowel syndrome. Curr Psychiatry Rep. 2004;6:210–215. doi: 10.1007/s11920-004-0066-0. [DOI] [PubMed] [Google Scholar]
- 8.Billings AG, Moos RH. Coping, stress, and social resources among adults with unipolar depression. J Pers Soc Psychol. 1984;46:877–891. doi: 10.1037//0022-3514.46.4.877. [DOI] [PubMed] [Google Scholar]
- 9.Folkman S, Lazarus RS. An analysis of coping in a middle-aged community sample. J Health Soc Behav. 1980;21:219–239. [PubMed] [Google Scholar]
- 10.Goodhand J, Rampton D. Psychological stress and coping in IBD. Gut. 2008;57:1345–1347. doi: 10.1136/gut.2008.154229. [DOI] [PubMed] [Google Scholar]
- 11.Esler M, Julius S, Zweifler A, Randall O, Harburg E, Gardiner H, et al. Mild high-renin essential hypertension. Neurogenic human hypertension? N Engl J Med. 1977;296:405–411. doi: 10.1056/NEJM197702242960801. [DOI] [PubMed] [Google Scholar]
- 12.Harburg E, Julius S, McGinn NF, McLeod J, Hoobler SW. Personality Traits and Behavioral Patterns Associated with Systolic Blood Pressure Levels in College Males. J Chronic Dis. 1964;17:405–414. doi: 10.1016/0021-9681(64)90101-8. [DOI] [PubMed] [Google Scholar]
- 13.Julius S. The psychophysiology of borderline hypertension. New York: Raven; 1981. [PubMed] [Google Scholar]
- 14.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 15.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 16.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl) 2012;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Oortmerssen GA, Benus I, Dijk DJ. Studies in wild house mice: Genotype-environment interactions for attack latency. Neth J Zool. 1985;35:155–169. [Google Scholar]
- 20.Kendler KS, Kessler RC, Heath AC, Neale MC, Eaves LJ. Coping: a genetic epidemiological investigation. Psychol Med. 1991;21:337–346. doi: 10.1017/s0033291700020444. [DOI] [PubMed] [Google Scholar]
- 21.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology (Berl) 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson KA, Stephen A, Beck SG, Valentino RJ. Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology. 2006;31:2449–2461. doi: 10.1038/sj.npp.1301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nava F, Calapai G, De Sarro A, Caputi AP. Interleukin-1 receptor antagonist does not reverse lipopolysaccharide-induced inhibition of water intake in rat. Eur J Pharmacol. 1996;309:223–227. doi: 10.1016/0014-2999(96)00352-4. [DOI] [PubMed] [Google Scholar]
- 28.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, et al. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 29.Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, et al. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry. 2012;17:49–61. doi: 10.1038/mp.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 31.Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borsody MK, Weiss JM. The effects of endogenous interleukin-1 bioactivity on locus coeruleus neurons in response to bacterial and viral substances. Brain Res. 2004;1007:39–56. doi: 10.1016/j.brainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Castanon N, Bluthe RM, Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology (Berl) 2001;154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- 35.Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 36.West CH, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12:627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 38.Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- 40.Gelfo F, Tirassa P, De Bartolo P, Croce N, Bernardini S, Caltagirone C, et al. NPY intraperitoneal injections produce antidepressant-like effects and downregulate BDNF in the rat hypothalamus. CNS neuroscience & therapeutics. 2012;18:487–492. doi: 10.1111/j.1755-5949.2012.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redrobe JP, Dumont Y, Fournier A, Quirion R. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26:615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- 42.Illes P, Finta EP, Nieber K. Neuropeptide Y potentiates via Y2-receptors the inhibitory effect of noradrenaline in rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:546–548. doi: 10.1007/BF00173217. [DOI] [PubMed] [Google Scholar]
- 43.Finta EP, Regenold JT, Illes P. Depression by neuropeptide Y of noradrenergic inhibitory postsynaptic potentials of locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:472–474. doi: 10.1007/BF00171093. [DOI] [PubMed] [Google Scholar]
- 44.Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlezon WA, Jr, Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacology & therapeutics. 2009;123:334–343. doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: A key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Pollier F, Sarre S, Aguerre S, Ebinger G, Mormede P, Michotte Y, et al. Serotonin reuptake inhibition by citalopram in rat strains differing for their emotionality. Neuropsychopharmacology. 2000;22:64–76. doi: 10.1016/S0893-133X(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 51.McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. The EMBO journal. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;184:128–138. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 53.Brambilla D, Franciosi S, Opp MR, Imeri L. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur J Neurosci. 2007;26:1862–1869. doi: 10.1111/j.1460-9568.2007.05796.x. [DOI] [PubMed] [Google Scholar]
- 54.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Frontiers in neuroendocrinology. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends in neurosciences. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 58.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O'Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 59.Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr Scand. 2001;103:226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 60.Watkins LR, Hansen MK, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of the proinflammatory cytokine, interleukin-1beta: molecular biology for non-molecular biologists. Life Sci. 1999;65:449–481. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 61.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 62.Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. European archives of psychiatry and clinical neuroscience. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- 63.Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain, behavior, and immunity. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 65.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annual review of immunology. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 67.Villiger PM, Terkeltaub R, Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992;149:722–727. [PubMed] [Google Scholar]
- 68.Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical & developmental immunology. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, et al. Pro-inflammatory biomakers in depression: treatment with venlafaxine. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2009;10:313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- 70.Davison GC, Williams ME, Nezami E, Bice TL, DeQuattro VL. Relaxation, reduction in angry articulated thoughts, and improvements in borderline hypertension and heart rate. Journal of behavioral medicine. 1991;14:453–468. doi: 10.1007/BF00845104. [DOI] [PubMed] [Google Scholar]
- 71.Shapiro D, Hui KK, Oakley ME, Pasic J, Jamner LD. Reduction in drug requirements for hypertension by means of a cognitive-behavioral intervention. American journal of hypertension. 1997;10:9–17. doi: 10.1016/s0895-7061(96)00258-0. [DOI] [PubMed] [Google Scholar]
- 72.Gloaguen V, Cottraux J, Cucherat M, Blackburn IM. A meta-analysis of the effects of cognitive therapy in depressed patients. J Affect Disord. 1998;49:59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- 73.Holroyd KA, O'Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson BW. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. JAMA. 2001;285:2208–2215. doi: 10.1001/jama.285.17.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henry JP, Liu YY, Nadra WE, Qian CG, Mormede P, Lemaire V, et al. Psychosocial stress can induce chronic hypertension in normotensive strains of rats. Hypertension. 1993;21:714–723. doi: 10.1161/01.hyp.21.5.714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.