Abstract
Cerebral toxoplasmosis is a frequent cause of focal brain lesions in the setting of immunodeficiency states, particularly the acquired immune deficiency syndrome (AIDS) and MR imaging is an important diagnostic modality to differentiate toxoplasmosis from tuberculoma, and primary central nervous system lymphoma with diverse therapeutic implications. Several imaging patterns have been described in cerebral toxoplasmosis. The “concentric target sign” is a recently described MRI sign on T2 weighted imaging of cerebral toxoplasmosis that has concentric alternating zones of hypo- and hyper intensities. It is believed to be more specific than the well known “eccentric target sign” in the diagnosis of cerebral toxoplasmosis and hence more useful in differentiation from other focal brain lesions in the context of AIDS. The concentric target sign, seen in deep parenchymal lesions is distinct from the surface based cortical “eccentric” target sign. The histopathological correlate of the latter has been recently described, but that of the concentric target sign is not known. In this study, we describe the neuropathological correlate of this “concentric target sign”, following post-mortem of a 40 year old man with AIDS associated cerebral toxoplasmosis. The concentric alternating zones of hypo/hyper/iso/and hyper intensities corresponded to zones of hemorrhage/fibrin rich necrosis with edema/coagulative compact necrosis/inflammation with foamy histiocytes admixed with hemorrhage forming the outer most zone respectively. The exclusive specificity of this sign in cerebral toxoplasmosis remains to be further elucidated.
Keywords: concentric target sign, MRI, T2 WI, toxoplasmosis, AIDS, neuropathology
Introduction
Opportunistic infections (OI) secondary to viruses, fungi and parasites are a common cause of mortality and morbidity in HIV infected individuals especially in resource limited countries (1). Toxoplasmosis, a common opportunistic human protozoan infection seen in immunocompromised patients, is the most frequent cause of intracranial mass lesions in patients with AIDS, accounting for 50-70% of all mass lesions in this population (2).
In Asia and sub-Saharan Africa, where the prevalence of HIV/AIDS is high, CNS involvement is most often secondary to opportunistic infections like tuberculosis, cryptococcal meningitis and cerebral toxoplasmosis and less commonly due to CNS lymphomas or HIV encephalitis (3,4).
In the archives of the HIV Registry of autopsied cases in the Human Brain Tissue Repository, at the Department of Neuropathology, in a tertiary care hospital, 190 cases of HIV/AIDS with neurological complications were autopsied between 1990–2011. Cryptococcal meningitis was the most frequent opportunistic infection (29.4%) followed by cerebral toxoplasmosis (23.6%) and tuberculous meningitis (18.9%). Unlike Western studies, CNS lymphomas were extremely infrequent.
Cerebral toxoplasmosis is a treatable opportunistic infection, following timely institution of appropriate drug therapy. However, differentiating this condition from close mimics such as primary CNS lymphoma and tuberculoma based on MR imaging features that often overlap is a challenge, with diverse therapeutic implications. Several imaging patterns have been described in cerebral toxoplasmosis of which, the ‘eccentric target sign’ on post contrast T1 weighted imaging, observed in less than 30% of cases, has been considered highly suggestive of toxoplasmosis (5). The histopathological correlate of this imaging feature has been recently described by our group (6).
A more recently described sign in cerebral toxoplasmosis is the “concentric target sign” on T2W MR imaging which has alternating concentric layers of T2 weighted hypo and hyper intensities, believed to be more specific for cerebral toxoplasmosis (7). Herein we describe the histopathological correlate of the ‘concentric T2 target sign’ by comparing the imaging findings with large whole mount histopathological sections of the lesions in an autopsied case of cerebral toxoplasmosis with AIDS.
Case Report
A 40 year old gentleman, driver by occupation was brought to the neurological emergency services of a tertiary care centre for neurological disorders,. He complained of fever of 7 days duration followed by alteration in sensorium for which he sought medical attention. He was a chronic alcoholic and smoker with history of promiscuous behaviour. At admission to casualty, he was in altered sensorium. His vital parameters were stable. He had oral thrush. Neurological examination revealed right sided hemiparesis with evidence of nuchal rigidity. Serology for HIV-1 tested positive and was subtyped C, using a subtype specific PCR, (8).
A cranial MRI performed on 1.5T Siemens Magnetom Vision system revealed multiple, well defined lesions involving bilateral thalami, left caudate, right lentiform nuclei, bilateral corona radiata and centrum semiovale, and mid brain (Fig.1a-c). The lesions in the bilateral superior frontal and cingulate gyrus involved the grey white junction and were hypo intense on T1WI and hyper intense on T2WI and FLAIR with perilesional edema (Fig.1c, arrow). Some of these also had central and eccentric hypo intensities on T2WI. The lesion in the left caudate head appeared heterogeneously hyper intense on T1WI (Fig.1a).
Fig 1.
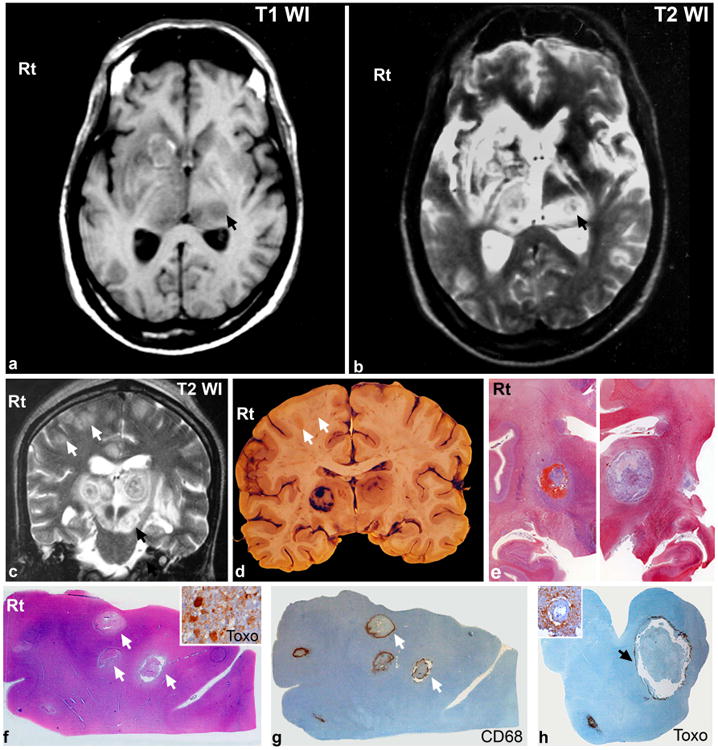
a,b: Axial T1WI and T2WI taken at the level of basal ganglia show multiple lesions involving the bilateral thalami, left caudate, right lentiform nucleus and left crus cerebri (arrow, b). Lesions in the thalamus appear hypointense on T1WI (a) with corresponding concentrically hypo and hyper intense zones on T2WI (“concentric target” sign) (b) with perilesional edema. Small punctate hyper intensities seen within the lesion on T1WI and ring like hyperintense left caudate lesion (a) suggests the presence of paramagnetic material/blood.
c: Coronal T2WI at the level of bilateral thalami and crus cerebri shows multiple lesions involving the bilateral thalami, and crus cerebri with perilesional edema. The thalamic lesions revealed “concentric target like” appearance with multiple layers of alternate concentric hypo- and hyper intensities with central hypointensity. Similar lesion also seen in left crus cerebri (black arrow). Smaller hyperintense lesions are noted in the bilateral superior frontal and cingulate gyrus at grey white junction (white arrows). Some of these have central/eccentric hypointensities.
d,e: Gross coronal slice of brain at same level as MRI revealed well circumscribed lesions in bilateral thalamus (d). The lesion on the right side had concentric zones of hemorrhage separated by pale necrotic zones, while the left was necrotizing with a thin outer and inner hyperaemic rim. Multiple smaller abscess like lesions (0.5 cms across) are seen along the grey white junction of the right superior frontal gyrus (d, arrows).
Large format whole mount histologic preparation from bilateral basal ganglia (e) taken at same level as lesions on MRI for comparison reveal large hemorrhagic and necrotizing lesions.
f-h: The lesions in right frontal cortex are organising abscesses with central zones of coagulative necrosis (f) and surrounding histiocytes (g). Tachyzoites and bradyzoites of T gondii at the periphery of lesion is seen by immunohistochemistry (f, inset). Note accumulation of tachzoites of T gondii seen rimming the lesion in crus cerebri on immunohistochemsitry (h). Inset reveals numerous ruptured tachyzoites surrounding inflamed vessels.
[e: Whole mount preparation, Masson trichromex8, f: HEx10, f, inset: Toxoplasma immunostain xObj.40, g: Cd68 immunostainx10, h: Toxoplasma immunostainx10, h,inset: Toxoplasma immunosatinxObj.20]
On coronal T2WI, the lesions involving bilateral thalami had “concentric target like” appearance with alternate hypo and hyper intensities (target sign) and central hypo intensity. Correspondingly, on T1W imaging, the right thalamic lesion had alternating concentric layers of central hyper intensity surrounded by hypo and hyper intensities (Fig.1a). The left thalamic lesion on T2WI had a central zone of patchy iso-hyperintensity with cap of hypointensity, enclosed by an outer alternate thin rim of hyper and hypointensity, with surrounding edema (Fig.1c). Axial FLAIR images (not included) revealed similar findings as T2WI with a target sign in the lesions in bilateral thalami and left cerebral crus of the midbrain (Fig.1c, arrow).
Based on MRI findings, a possibility of cerebral toxoplasmosis was entertained and he received antitoxoplasma regimen, mannitol, and steroids to lower the intracranial pressure. He however continued to deteriorate and succumbed within two weeks of admission.
Consent for partial autopsy limited to examination of brain alone was obtained from the close relatives. The study was approved by the Institutional Scientific Ethics Committee. At autopsy, the brain was diffusely swollen with bilateral tonsillar and uncal herniation. The leptomeninges covering the surface were mildly hazy, though no tubercles were noted. On serial coronal slicing, multiple large parenchymal organizing abscesses with central zones of waxy necrosis and surrounding hyperemia were noted in left caudate and globus pallidum abutting onto the internal capsule and right caudate and putamen corresponding to the lesions detected on MR imaging.
In the thalamus bilaterally, corresponding to the lesions displaying concentric target sign on T2WI, two well circumscribed lesions were seen (approx 2cms across) (Fig.1d). The lesion in the right thalamus had concentric zones of hemorrhage separated by pale necrotic zones. More medially, a small punched out necrotic lesion was noted close to the third ventricle. The left thalamic lesion had a large central zone of necrosis with a cap of hyperaemia and larger surrounding rim of hemorrhage (Fig.1d). Whole mount histologic preparation from bilateral thalamic lesions corresponding to MRI were studied for point to point correlation (Fig.1e)
A large circumscribed necrotic lesion was seen in the left cerebral peduncle, destroying the substantia nigra with multiple smaller lesions (0.5 cms across) along the grey white junction of the right superior frontal, cingulate gyrus, superior parietal lobule and bilateral occipital cortices lacking grossly visible hemorrhage (Fig.1d). These lesions corresponded to the “organising abscess stage” with central coagulative necrosis and surrounding histiocytes (Fg.1f,g). Immunohistochemistry revealed tachyzoites and bradyzoites of T gondii (Fig.1f, inset) forming a rim around the peduncular lesion (Fig.1g, & inset).
Histopathological findings on whole mount sections of bilateral thalamic lesions were matched with concentric target sign on T2WI for pathological correlation (Fig.1e, 2a-c). In the right thalamic lesion, a small central zone of fresh hemorrhage surrounded an inflamed vessel (Fig.2b,d,e) with outer most band of hemorrhage (Fig.2b-d,h) that corresponded to intensely hypo intense core and outermost rim (zones 1 & 5 in Fig.2a). This was enclosed by zones of fibrin rich necrosis with thrombosed small venules exuding fibrin and edema fluid from the damaged walls (Fig.2d,f) seen as a hyper intense zone on T2WI (Fig.2a– zone 2). Outer to this, the iso intense zone 3 corresponded to a band of compact coagulative necrosis (Fig.2a,d,g). This was walled in by a broad band of foamy histiocytes (zone 4) followed by fresh zone of hemorrhage (zone 5, (Fig.2 d,h) representing alternating hyper and hypo intense bands on T2W imaging (Fig.2a, zones 4 &5).
Fig.2. Radiopathological correlation (right thalamic lesion).
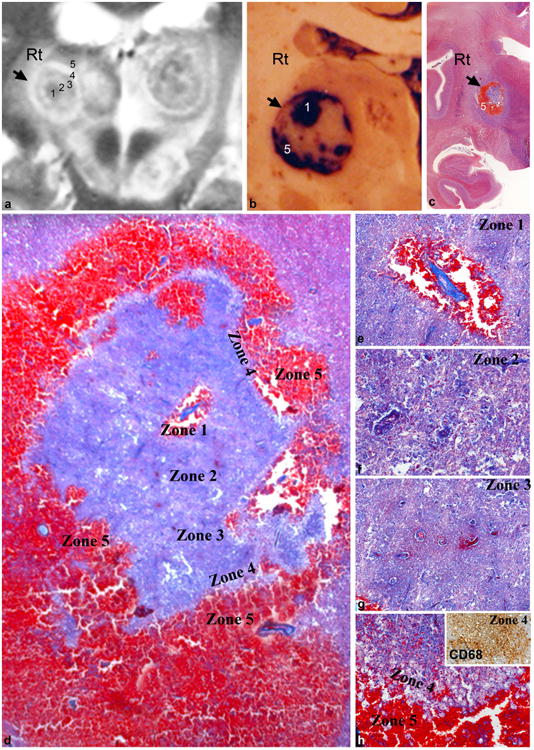
Gross and histopathological findings on whole mount sections of thalamic lesion (b,c), were correlated with imaging features (a, magnified view of Fig.1c). The central and outermost hypointense zones on T2WI (a- zones 1 and 5) corresponded to foci of fresh hemorrhage (b,d,e,h). Alternating hyperintense bands on T2WI (zones 2 and 4 in a) corresponded to fibrin rich necrosis with thrombosed venules, and edema fluid or foamy histiocytic infiltration (d,f,g). The intervening isointense zone 3 on MRI (a) was a band of compact coagulative necrosis (d,g).
[c: Whole mount Masson trichrome x8, d: Masson trichrome xObj.1.6, e,h: Masson trichrome xObj.20, f,g: Masson trichrome xObj.20]
The left thalamic lesion (Fig.3a-i) had central zone of fibrin rich necrosis (Fig.3b) and occlusive hypertrophic vessels with thrombosed lumina and fibrin exuding from the vessels (Fig.3d,e) corresponding to innermost zone 1 that was iso-hypo intense on T2WI (Fig.3a). This was capped by a zone of fresh RBCs surrounding ruptured and thrombosed veins (Fig.3d,f) seen as a hypo intense cap on T2WI and zone of hyperaemia on gross examination (Fig.3a,– zone 2). These vessels were thin walled with immune complex deposition on the necrosed walls and fibrin extravasating from them (Fig.3f). A zone of fibrin rich necrosis with thrombosed vessels and edema (Fig.3d,g) represented zone 3 that was hyper intense on T2WI (Fig.3a). The next zone 4 had compact coagulative necrosis with nuclear and granular cytoplasmic debris of dead cells punctuated by several hyperplastic vessels demonstrating occlusive arteritis (Fig.3a,d,h, isointense on T2WI). The outer most hyperintense band on T2WI (Fig.3a, zone 5) corresponded to a band of foamy histiocytes, immunolabelled by CD68 (zone 5, Fig.3c,i). Variable degree of edema was noted in the surrounding brain, at places forming a thin cleft separating the lesion from the adjacent parenchyma. Reactive astrocytic cuff around the lesion was surprisingly mild and only occasional tachyzoites forms of T gondi could be detected by tachyzoite specific antibodies.
Fig.3. Radiopathological correlation (left thalamic lesion).
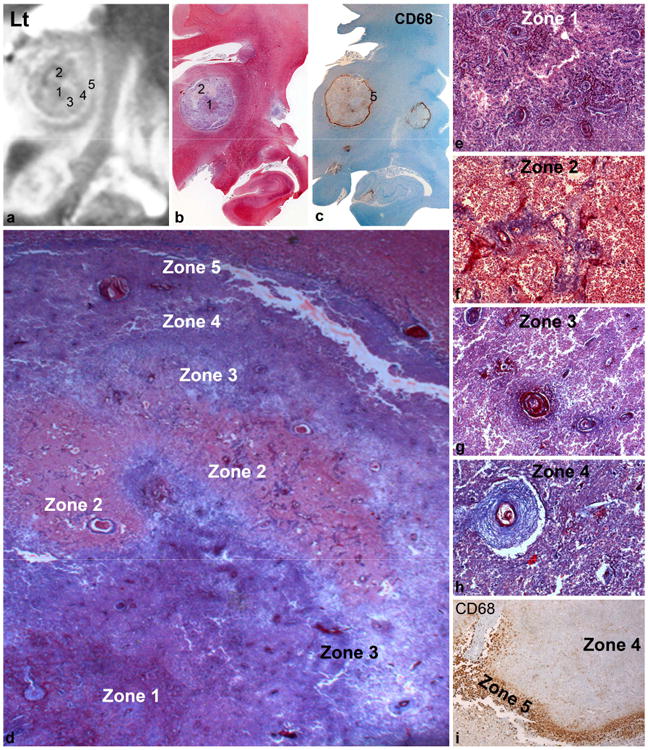
The left thalamic lesion had central zone of fibrinoid necrosis with RBC (b,d,e) corresponding to central iso-hypointense zone 1 on MRI (a). This was capped by a zone of fresh hemorrhage surrounding ruptured veins (b,d,f) seen as hypointense cap on T2WI (a– zone 2). Alternate zones of hyperintensity (zones 3&5 in a) corresponded to fibrin rich necrosis with edema (zone 3, d&g) and ring of foamy histiocytes highlighted by immunostaining with antibody to CD68 (zone 5, c&i). The intervening isointense zone (zone 4, a) had compact coagulative necrosis with thick walled hyperplastic vessels demonstrating occlusive endarteritis (zone 4, d&h).
[b: Whole mount preparation Masson trichrome x8, c: CD68 whole mount x8, d: Masson trichrome xObj.x1.6, e-h: Masson trichrome xObj.20, i: Immunostaining CD68xObj.10]
Discussion
Neurological complications secondary to opportunistic infections develop in upto 40% of patients with HIV/AIDS. CNS Toxoplasmosis is the most common cause of solitary or multiple intracerebral lesions in AIDS patients followed by CNS lymphoma (9).
The incidence of CNS toxoplasmosis varies in different studies. The differences in prevalence are probably reflected by the seroprevalence of toxoplasmosis in the general population. For instance in the USA, seroprevalence is 15%, in contrast to more than 50% - 75% in some European Countries (10), and 20.3% in a study from India (11). In an autopsy based study, cerebral toxoplasmosis was found to be the most common cause of focal brain lesions in HIV/AIDS (4).
Differentiating cerebral toxoplasmosis from primary CNS lymphoma and tuberculosis is a challenging clinical and radiographic dilemma with diverse therapeutic implications (2,12). Noninvasive diagnostic modalities such as MR spectroscopy and SPECT imaging were employed to distinguish from primary CNS lymphoma or tuberculoma with varied results (13,14). Empirical anti-toxoplasmosis therapy for distinguishing toxoplasmosis from lymphoma has its own disadvantages, since toxoplasma lesions may take upto 6 weeks for resolution (15). Moreover, in a significant number of patients, allergic reactions or hematologic toxicity can occur with anti-toxoplasmosis therapy (16). Brain biopsy for ascertaining diagnosis is generally avoided because of the associated morbidity, and sampling errors. Hence non-invasive diagnostic modalities assume importance.
On neuroimaging, several characteristic patterns are described in cerebral toxoplasmosis that provide a diagnostic clue (Table 1). The most common, is the post contrast T1W “eccentric target sign” that has three alternating zones: an innermost enhancing core that is eccentric, an intermediate hypo intense zone and an outer peripheral hyper intense enhancing rim. This produces an annular enhanced area with a central nodule that is often eccentric, hence termed ‘eccentric target sign’. Though considered highly suggestive of toxoplasmosis, it is found in less than 30% of cases (17). The neuropathological correlate of this eccentric target sign has been recently described, (6). These lesions are usually observed close to cortical surface enclosing both the lips of cortical ribbon, with the sulcus in between through which thickened, inflamed vessels traverse to produce the eccentric innermost enhancing core. The intermediate hypointense and peripheral enhancing rim are caused by compact zone of necrosis and surrounding ring of histiocytes with inflammatory granulation tissue respectively.
Table 1. CT/MR Imaging characteristics of “target signs” in various conditions.
| Types of target signs | Conditions | Centre | Periphery | Pathology correlate |
|---|---|---|---|---|
| CT contrast: two zone target | Tuberculoma (22) | Hypodense | Enhancing border | Centre: caseation Periphery: histiocytes + leaky inflamed vessels |
| Metastases (23,24) | Hypodense | Enhancing border due to leaky blood vessels | Centre: inspissated mucin Periphery: leaky vessels |
|
| MRI T2WI: two zone target | Tuberculoma (25,26) | Hypo intense | Hyper intense | Centre: caseation Periphery: histiocytes + leaky inflamed vessels |
| Toxoplasmosis (7) | Hyper intense | Hypo intense | Centre: fibrin + edema rich necrosis Periphery: closely packed histiocytes |
|
| MRI T1WI (post contrast): two zone target | Toxoplasmosis (27) | Iso/hypo intense | Ring enhancement | Not known |
| MRI T1WI (non enhanced): two zone target | Toxoplasmosis (28) | Hypo intense | Iso intense | Not known |
| MRI T1WI (post contrast): 3 zone target (eccentric/central target sign) | Toxoplasmosis (17) | Enhancing central/eccentric core | Intermediate: hypo intense Outer most: enhancing hyper intense |
Organizing abscess stage Enhancing core: inflamed vessels in sulcus running through centre of lesion Intermediate hypo intensity: compact necrosis Enhancing periphery: inflammation, endarteritis (6) |
| MRI T2WI/FLAIR: 3 zone target (Concentric T2 target sign) Contrast pattern: not known | Toxoplasmosis (7) | Hypo intense | Intermediate: hyper intense Outer: iso Outer most: hypo intense |
Central hypo intensities: hemorrhage Intermediate hyper intensities: fibrin rich necrosis Outer iso – compact necrosis Outermost hypo intensity – edema (present report) |
| MRI T2WI/FLAIR: 3 zone target (T2 target sign) T1 and Contrast: lamellar rings |
Balo's concentric sclerosis (18) | Hyper intense | Alternating hypo intense | Alternate histiocyte rich foci of demyelination and remyelination |
A new T2W/FLAIR target sign with reverse zonation (a hypo intense core, an intermediate hyper intense and a peripheral hypo intense zone) was recently described by Masamed and colleagues as a more specific sign diagnostic of toxoplasmosis (7). In a review of 14 cases from their records, the authors found the T2W/FLAIR target sign in isolation was seen more frequently in cases of cerebral toxoplasmosis (29%) than the post contrast T1 eccentric target sign (7%) while majority had either or both signs (71%). The two signs were only rarely seen in the same lesions suggesting that they reflect different pathological stages of toxoplasma lesions in evolution, varying in the extent of hemorrhage.
The histological correlate of the concentric T2 target sign in cerebral toxoplasmosis is presented in this study for the first time. The central T2 hypointensities bilaterally corresponded to hemorrhage on histologic examination, and the alternating bands of hyper intensities due to fibrin rich necrosis with edema, and histiocytes. Inspissated coagulative necrosis lacking free water, and shortened T2 relaxation times produced iso-intensities on MRI. This sign has hitherto not been recorded in any other AIDS associated CNS lesions reflecting higher specificity of concentric target sign for toxoplasmosis (7).
Balo's concentric sclerosis is the only non infective condition with MRI features of lamellar hypoisointense concentric rings on T1-weighted, and whorled hyper intense concentric rings on T2-weighted. This however has a central hyper intense core with concentric rings of enhancement that correspond to alternate rings of demyelination and remyelination whereas in toxoplasmosis, the core is hypo intense (18). A target sign has recently been described in diffusion weighted imaging secondary to cerebral aspergillosis in immunocompromised patients, acute necrotizing encephalopathy and Balo's concentric sclerosis (19).
Toxoplasma infection of the CNS produces three morphologic types of pathologic lesions: necrotizing, organizing and chronic abscesses based on the host's immune response to the protozoan and the imaging patterns reflect this temporal evolution (20). The initial acute necrotizing abscess stage consist of acute inflammatory granulation tissue, zones of necrosis and petechial haemorrhages with numerous free-living tachyzoites and encysted organisms. In about 2–4 weeks, a fibrous capsule with endarteritic vessels forms around the necrotic centre that is free of parasites, heralding the organising abscess stage that resembles tuberculomas. The third stage, progressing to chronic stage, has vascular fibrous scar with paucity of toxoplasma tachyzoites. On T1-weighted imaging, necrotizing lesions appear hypo-intense to the gray matter whereas, presence of focal hyper intensity is related to haemorrhage or calcification (21). T2-weighted images reveal variable signal intensities, being hyper or hypo intense relative to the gray matter as the lesion progresses.
Several “target signs” have been described both on CT scans and MRI (Table 1). The target sign initially described on CT scans was considered characteristic of tuberculoma (22), and in some cases of metastatic carcinoma (23,24). In both instances, the central hypo dense area corresponded to caseous necrosis or myxoid matrix and the contrast enhancing border was caused by leakage of the dye from proliferating blood vessels with defective blood barrier or an active macrophage response.
The “target sign” on MRI, on the other hand is relatively rare even in cases of CNS tuberculoma (25). Wasay et al in their study of 100 cases of tuberculomas found the “target sign” in only two cases (26). In tuberculomas, the target has a T2W hypo intense center attributed to compact caseous necrosis with edematous border that is T2 hyper intense due to easy movement of water molecules. In contrast, in toxoplasmosis, the target sign on T2 has bright centre with dark rim due to fibrin rich central necrosis and edema with wall of closely packed histiocytes (7). Many of these features are frequently seen in clinical practice, but the pathobiological basis is not elucidated.
The three zone target signs on MRI appear to be more specific. Occasionally the T1W target has only two zones with central iso/hypo intensity and surrounding ring enhancement (27). Miguel and co-workers aso reported a two- zone target on non-enhanced MR with central hypo intensity and peripheral iso intensity. The pathologic correlate of these signs has however not been reported (28).
Brightbill and colleagues, analysing 27 patients of cerebral toxoplasmosis discovered three distinct imaging patterns that varied depending on treatment duration (29). The presence of T2 hyper intensity was seen in acute initial stages in absence of treatment, with transition from mixed intensity to T2 isointensity following long term therapy for weeks to months. The authors hypothesise that changes in T2 signal intensities reflect change from liquefactive necrosis in acute stage to coagulative necrosis producing T2 shortening with chronicity. Presence of hemorrhage/calcification is not mentioned. Other workers (21) reported hyperdensity on CT or T1W hyper intensities on MR imaging following 6 months or more of treatment that histologically corresponded to hemorrhage or calcification.
In the present case, the patient had received antitoxoplasma regime for 12 days. This might have induced fresh hemorrhage following immune reconstitution induced vasculitis/venulitis. In the original report of Masamed and colleagues, details of treatment history are not available for correlation. Further studies are essential to ascertain the specificity of this new sign and determine if it is a treatment induced change.
In the present case, although we have post-mortem histopathology for correlation with MRI changes, several factors need to be taken into account. The interval between MRI and post-mortem was 13 days. This could be responsible for larger zones of hemorrhage seen post-mortem compared to antemortem MRI although the interval may not be sufficiently long to induce fibrous scarring or endarteritis. Secondly, there is a mild discordance in plane of MRI and the corresponding anatomical slice of the brain. While both these factors may preclude an absolute point-to-point correlation, we believe that the information derived is still valuable. For more precise correlation of MRI images with histology, newer imaging modalities such as volumetric scan or MP RAGE (Magnetization Prepared Rapid Gradient Echo) which generates reformatted images from thin section 3D imaging in T1WI, T2WI and FLAIR sequences will be useful to correlate with histology.
The present study demonstrates histopathological correlate of the concentric target sign of cerebral toxoplasmosis on T2 weighted MR sequences. These studies provide insight into varying MR imaging characteristics correlating with tissue response during the evolution of the pathology. This also offers a phenomenological understanding of similar MR imaging features in diverse pathological entities, at times causing diagnostic dilemmas.
Acknowledgments
The authors wish to acknowledge assistance of Mr. H.N. Nagesh, Mrs. Rajyasakti, Mr. Prasanna Kumar, and Mr. Shivaji Rao, Human Brain Tissue Repository (Brain Bank), for assistance with whole mount histological preparations and immunohistochemistry as well as Mr. N. Manjunath, Department of Neuropathology for assistance with microphotography and preparation of montages.
Funding source: This publication was partly supported by a subaward from The Johns Hopkins University, with funds provided from National Institute of Neurological Disorders and Stroke (NINDS) [Grant no: 1RO1NS055628-01A2]. Its contents of the study are solely the responsibility of the authors and do not represent the official view of NINDS or JHU.
References
- 1.Lane HC, Laughon BE, Falloon J, et al. NIH conference: Recent advances in the management of AIDS-related opportunistic infections. Ann Intern Med. 1994;120:945–55. doi: 10.7326/0003-4819-120-11-199406010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Smirniotopoulos JG, Koeller KK, Nelson AM, Murphy FM. Neuroimaging--autopsy correlations in AIDS. Neuroimaging Clin N Am. 1997 Aug;7(3):615–37. [PubMed] [Google Scholar]
- 3.Satishchandra P, Nalini A, Gourie-Devi M, et al. Profile of neurological disorders associated with HIV/AIDS from Bangalore, South India (1989-1996) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- 4.Shankar SK, Mahadevan A, Satishchandra P, et al. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res. 2005 Apr;121(4):468–88. [PubMed] [Google Scholar]
- 5.Ramsey RG, Gean AD. Neuroimaging of AIDS. I. Central nervous system toxoplasmosis. Neuroimaging Clin N Am. 1997 May;7(2):171–186. [PubMed] [Google Scholar]
- 6.Kumar GG, Mahadevan A, Guruprasad AS, et al. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31:1469–1472. doi: 10.1002/jmri.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masamed R, Meleis A, Lee EW, Hathout GM. Cerebral toxoplasmosis: case review and description of a new imaging sign. Clin Radiol. 2009;64:560–563. doi: 10.1016/j.crad.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Siddappa NB, Dash PK, Mahadevan A, et al. Identification of subtype C human immunodeficiency virus type 1 by subtype-specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol. 2004;42:2742–2751. doi: 10.1128/JCM.42.6.2742-2751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy RM, Rosenbloom S, Perrett LV. Neuroradiology findings in AIDS: review of 200 cases. Am J Roentgenol. 1986;147:977–983. doi: 10.2214/ajr.147.5.977. [DOI] [PubMed] [Google Scholar]
- 10.Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK CDC. National Institutes of Health; Infectious Diseases Society of America. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep. 2004;53(RR-15):1–112. [PubMed] [Google Scholar]
- 11.Sundar P, Mahadevan A, Jayshree RS, Subbakrishna DK, Shankar SK. Toxoplasma seroprevalence in healthy voluntary blood donors from urban Karnataka. Indian J Med Res. 2007;126:50–55. [PubMed] [Google Scholar]
- 12.Dina TS. Primary central nervous system lymphoma versus toxoplasmosis in AIDS. Radiology. 1991;179:823–828. doi: 10.1148/radiology.179.3.2027999. [DOI] [PubMed] [Google Scholar]
- 13.Skiest DJ, Erdman W, Chang WE, Oz OK, Ware A, Fleckenstein J. SPECT thallium-201 combined with Toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect. 2000;40:274–281. doi: 10.1053/jinf.2000.0664. [DOI] [PubMed] [Google Scholar]
- 14.Chinn RJ, Wilkinson ID, Hall-Craggs MA, et al. Toxoplasmosis and primary central nervous system lymphoma in HIV infection: diagnosis with MR spectroscopy. Radiology. 1995;197:649–654. doi: 10.1148/radiology.197.3.7480733. [DOI] [PubMed] [Google Scholar]
- 15.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 16.Leport C, Raffi F, Matheron S, et al. Treatment of central nervous system toxoplasmosis with pyrimethamine and sulfadiazine combination in 25 patients with acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1988;84:94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay R, Gerenia GK. CNS complications of AIDS: CT and MRI findings. Am J Radiol. 1988;151:449–454. doi: 10.2214/ajr.151.3.449. [DOI] [PubMed] [Google Scholar]
- 18.Chen CJ, Chu NS, Lu CS, Sung CY. Serial magnetic resonance imaging in patients with Balo's concentric sclerosis: natural history of lesion development. Ann Neurol. 1999;46:651–656. doi: 10.1002/1531-8249(199910)46:4<651::aid-ana15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Finelli PF, Gleeson E, Ciesielski T, Uphoff DF. Diagnostic role of target lesion on diffusion-weighted imaging: a case of cerebral aspergillosis and review of the literature. Neurologist. 2010;16:364–367. doi: 10.1097/NRL.0b013e3181b47001. [DOI] [PubMed] [Google Scholar]
- 20.Navia BA, Petito CK, Gold JWM, Cho E, Jordan BD, Price RW. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1088;19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 21.Revel MP, Gray F, Brugieres P, Geny C, Sobel A, Gaston A. Hyperdense CT foci in treated AIDS toxoplasmosis encephalitis: MR and pathologic correlation. J Comput Assist Tomogr. 1992;16:372–375. doi: 10.1097/00004728-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 22.van Dyk A. CT of intracranial tuberculomas with specific reference to the “target sign”. Neuroradiology. 1988;30:329–336. doi: 10.1007/BF00328184. [DOI] [PubMed] [Google Scholar]
- 23.Kong A, Koukourou A, Boyd M, Crowe G. Metastatic adenocarcinoma mimicking ‘target sign’ of cerebral tuberculosis. J Clin Neurosci. 2006;13:955–958. doi: 10.1016/j.jocn.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Bargalló J, Berenguer J, García-Barrionuevo J, Ubeda B, Bargalló N, Cardenal C, Mercader JM. The “target sign”: is it a specific sign of CNS tuberculoma? Neuroradiology. 1996;38:547–550. doi: 10.1007/BF00626095. [DOI] [PubMed] [Google Scholar]
- 25.Enzmann DR, Brant-Zawadzki M, Britt RH. CT of central nervous system infections in immunocompromised patients. Am J Neuroradiol. 1980;1:239–243. doi: 10.2214/ajr.135.2.263. [DOI] [PubMed] [Google Scholar]
- 26.Wasay M, Kheleani BA, Moolani, et al. Brain CT and MRI Findings in 100 Consecutive Patients with Intracranial Tuberculoma. J Neuroimaging. 2003;13:240–247. [PubMed] [Google Scholar]
- 27.Osborne AG. Diagnostic Neuroradiol. St Loius, MO: Mosby; 1994. Infections of the brain; pp. 698–699. [Google Scholar]
- 28.Miguel J, Champalimaud JL, Borges A, Chorão M, Branco G, Doroana M, Medina E. Cerebral toxoplasmosis in AIDS patients, CT and MRI images and differential diagnostic problems. Acta Med Port. 1996;9:29–36. [PubMed] [Google Scholar]
- 29.Brightbill TC, Post MJ, Hensley GT, Ruiz A. MR of Toxoplasma encephalitis: signal characteristics on T2- weighted images and pathologic correlation. J Comput Assist Tomogr. 1996;20:417–422. doi: 10.1097/00004728-199605000-00019. [DOI] [PubMed] [Google Scholar]


