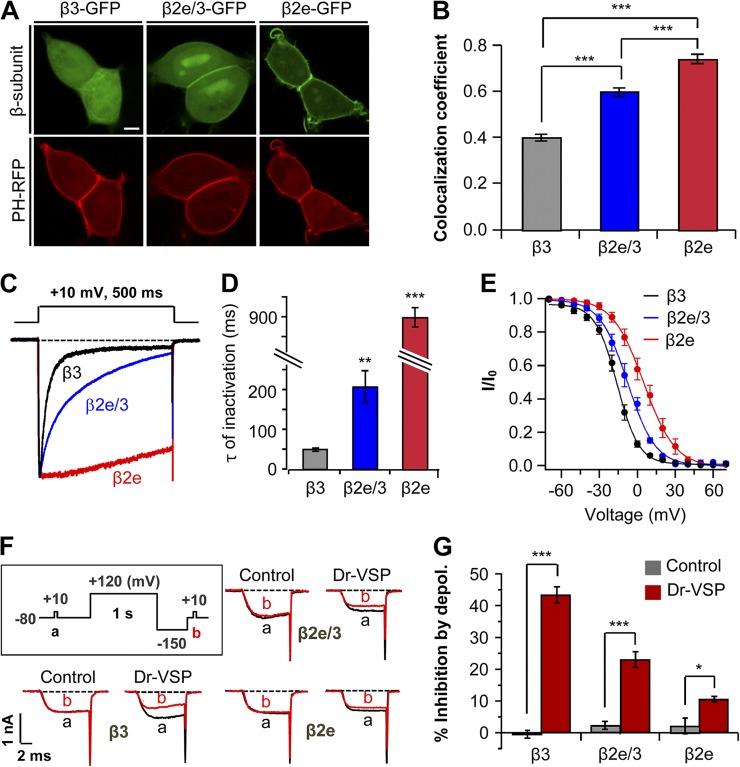
Figure 3.
Subcellular localization of β subunits affects inactivation and PIP2 sensitivity of CaV channels. (A) Confocal images of cells expressing β3-GFP, chimeric β2e/3-GFP (β3 subunit containing β2e N-terminal 23 amino acids), and β2e-GFP with membrane PIP2 probe PH-PLCδ-RFP (PH-RFP). Bar, 5 µm. (B) Quantification of plasma membrane localization of β3-GFP, chimeric β2e/3-GFP, and β2e-GFP subunits. ***, P < 0.001. (C) Effect of membrane-anchored β2e/3-GFP on the inactivation of CaV2.2 currents. Currents were measured during 500-ms test pulses to 10 mV in cells expressing CaV2.2 channels with β3, β2e/3-GFP, or β2e subunits. (D) Summary of time constant (τ) for CaV2.2 current inactivation with β3 (n = 4), β2e/3 (n = 4), and β2e (n = 5) subunits. **, P < 0.01; ***, P < 0.001, compared with β3. (E) Voltage dependence of normalized steady-state inactivation for CaV2.2 channels with β3 (n = 5), chimeric β2e/3 (n = 6), and β2e (n = 6) subunits. Normalized data were plotted against the conditioning potential. (F) Test protocol for depolarizing pulse and current inhibition of CaV2.2 channels with β3, β2e/3 and β2e subunits by Dr-VSP. The currents before (a) and after (b) a depolarizing pulse to 120 mV were superimposed in control and Dr-VSP transfected cells. (G) Summary of CaV2.2 current inhibition by membrane depolarization in control and Dr-VSP-expressing cells. For control, β3 (n = 5), β2e/3 (n = 4) or β2e (n = 5); for Dr-VSP, β3 (n = 4), β2e/3 (n = 4) or β2e (n = 5). * P < 0.05, *** P < 0.001, compared with control. Data are mean ± SEM.