Abstract
Inflammatory Bowel Disease (IBD) is nonspecific inflammation in the intestinal track, including Ulcerative Colitis (UC) and Crohn’s disease (CD). The incidence of IBD has increased significantly, with its numerous rising up to five million globally, more than 1,700,000 in China. Pathological character of IBD is the inflammation of intestinal mucosa and intestinal fibrosis. Although the pathogenesis of the disease has not yet been fully clarified, some evidence suggests that excessive intestinal inflammation reaction, intestinal barrier impairment and abnormal immune response can initiate IBD. As research continues, some of them have provided new insights toward understanding of Rho kinase signal pathway function at the occurrence and development of IBD. This review aims to summarize the general principles of Rho kinase signal pathway in the pathological procedure of IBD.
Keywords: Rho/Rho kinase, IBD, intestinal barrier, intestinal fibrosis
Rho/Rho kinase signal pathway
The Rho/Rho kinase signal pathway is ubiquitously expressed across the species, ranging from yeast to human. Rho kinase is a subgroup of the Ras superfamily of 20-to 30-KD GTP-binding proteins that have been shown to regulate a wide spectrum of cellular functions. In Rho family, RhoA, Rac and Cdc42, which are regarded as the core molecules that induce stress fibers to form and regulate cellular adherence by reconstructing cytoskeleton in response to extracellular growth factors, are deeply researched [1]. ROCK (Rho-associated kinase), which belongs to the member of the family of serine/threonine protein, is an acknowledgeable important signal molecule in the downstream of the Rho kinase signal pathway. It mainly includes two types: ROCK1 and ROCK2 [2]. ROCK1 is widely expressed in various tissues except the central nervous system; ROCK2 is mainly expressed in the central nervous system. Like all members of the Ras superfamily, the Rho kinase functions as molecular switches, cycling between an inactive GDP-bond form and an active GTP-bond form [3]. Extracellular signals trigger the activity of Rho, which then binds to Rho kinase and change GDP-Rho into GTP-Rho. Activated Rho kinase conducts signals to ROCK and phosphorylates the myosin light chain (MLC), leading to an accumulation of the phosphorylated form of MLC (pMLC), and then changing the cytoskeleton. Y-27632 is the unique inhibitor of ROCK that can inhibit the cell biology behavior induced by Rho signal pathway [4] (Figure 1). Numerous basic experiments suggest that Rho kinase signal pathway plays a critical role in diseases’ occurrence and development, such as heart disease [5], pancreas fibrosis [6] and pulmonary hypertension [7]. As research continues, it is believed that Rho kinase is related to intestinal diseases, IBD is one of them.
Figure 1.
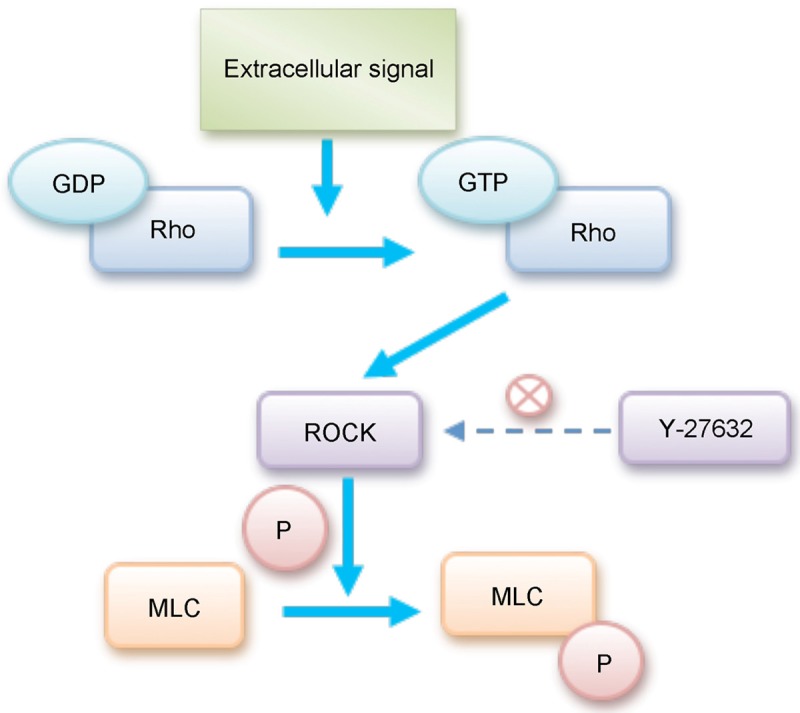
Regulation between ROCK and MLC. Extracellular signals initiate the activity of Rho, which then binds to Rho kinase and change GDP-Rho into GTP-Rho. Activated Rho kinase phosphorylates MLC, leading to an accumulation of pMLC. With the increasing of pMLC, it enhances stress fibrosis formation and rearranges F-actin cytoskeleton.
Intestinal barrier damage and immune activation in IBD
Intestinal epithelial integrity and immune isolation
Intestinal barrier is composed of three layers-the slime layer, the epithelial layer, and the basement membrane, which produces a complete biological barrier that isolates bacteria and antigens of the intestinal tract from immune cells in order to avoid immune response [8,9]. Of the three layers, cellular junction (Figure 2; [10]) which consists of tight junction (TJ), adherens junction (AJ) and desmosome in mucosa layer is the critical one. TJ is a multiple protein complex located at the apical ends of the lateral membrane of intestinal epithelial cells in keeping intestinal barriers integrality [10]. The TJ complex consists of transmembrane proteins [11-13] such as occluding, claudin, junction associated molecule (JAM) and intracellular proteins [14] such as zonula occludens (ZO).The transmembrane proteins interact with the intracellular proteins, which in turn create a permselective barrier in preventing bacteria and antigens from activating immune cells located in laminae propria. Simultaneously, TJ also plays an important role in intercellular substance transportation and maintenance of intestinal epithelial differentiation and recovery. Alteration of intestinal tight junction is regulated by myosin light chain (MLC) [15]. ROCK, myosin light chain kinase (MLCK) and other phosphorylated kinase can phosphorylate MLC to promote tight junction proteins (including transmembrane proteins and intracellular proteins) depredated in cells, ultimately inducing intestinal barrier damage and mucosal permeability impairment [16]. In addition, ROCK can also cause the contraction of F-actin cytoskeleton to regulate intestinal barrier integrity [17].
Figure 2.
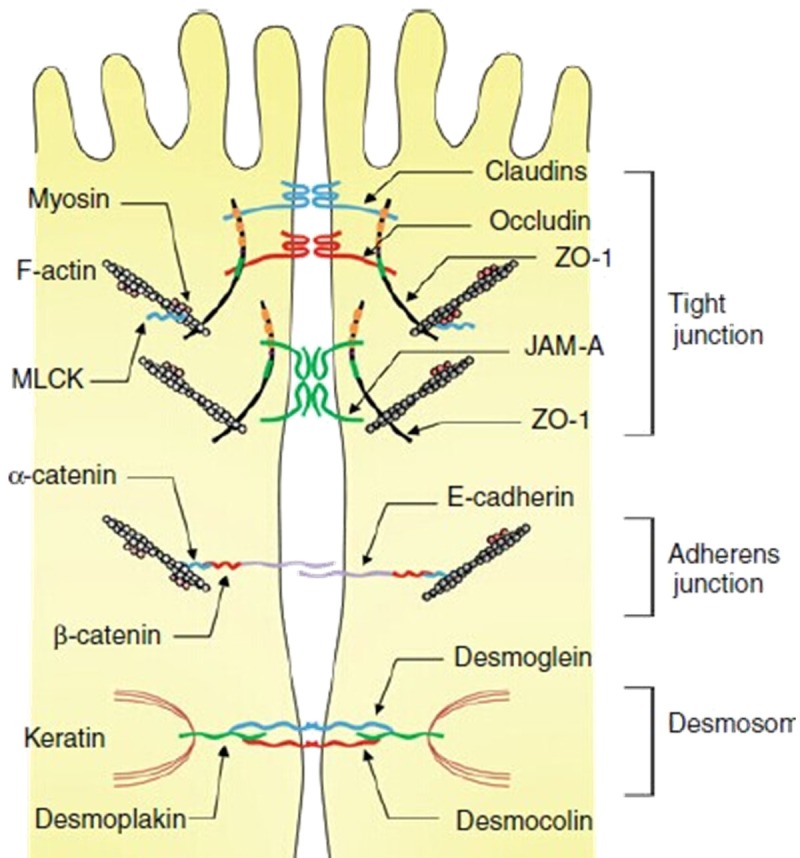
Cellular junctions in intestinal epithelium. It consists of TJ, AJ and desmosome. The TJ complex comprises transmembrane proteins such as occluding, claudin, JAM and intracellular proteins like ZO. The MLCK and F-actin can be regulated by Rho kinase.
Rho kinase signal pathway in intestinal barrier damage and immune activation
Accumulating evidence from basic science and clinical studies demonstrates three essential factors for IBD pathogenesis: disruptions of the intestinal barrier, exposure of the luminal content to mucosal immune cells and an abnormal immune response [18-21] (Figure 3). In IBD patients, it is suggested that expression of TJ proteins decreased obviously, but there was no difference between non-inflammatory intestinal tissue in IBD patients and healthy patients, indicating that abnormal expression of TJ proteins mainly located in inflammatory intestinal tissues [22]. Therefore, excessive inflammatory response in the intestinal tract destroys the expression and distribution of tight junction proteins. In CD, we can find that numerous inflammatory cytokines express in intestinal mucosa cells [23-31], including TNF-α, γ-IFN, Interleukin-1 (IL-1), Interleukin-1β (IL-1β), Interleukin-13 (IL-13). All of cytokines regarded as extracellular signals influence the expression of tight junction proteins, thus interfering cellular junction structure and changing intestinal integrity via Rho kinase mediated F-actin cytoskeleton regulation. Simultaneously, increased expression of γ-IFN [25] in IBD not only can inhibit the expression of TJ associated proteins like Occludin, Claudin-1/4, but increase the regulative proteins of TJ, such as ROCK, MLCK, which can make TJ proteins depredated [32]. TNF-α can also coordinate with IFN-γ to increase TJ regulative proteins of intestinal mucosa to change the intestinal barrier [33]. Furthermore, Segain’s experiment [34] provided the direct evidence that increased activation of RhoA was found in inflamed colonic mucosa of patients with CD and of rats with 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis. Oral administration of Y-27632 in rats significantly reduced the colonic inflammation via nuclear factor kappa B inhibition. It is clarified that radiation-induced enteropathy is associated with microvascular injury and epithelial barrier dysfunction [35]. Rho kinase activity constituted an important signaling mechanism in leucocyte-platelet-endothelium interaction, formation of cytokinase and reactive oxygen species, neutrophil accumulation. We deduce that this mechanism may have a similar effect in IBD, but more long-term studies are needed.
Figure 3.
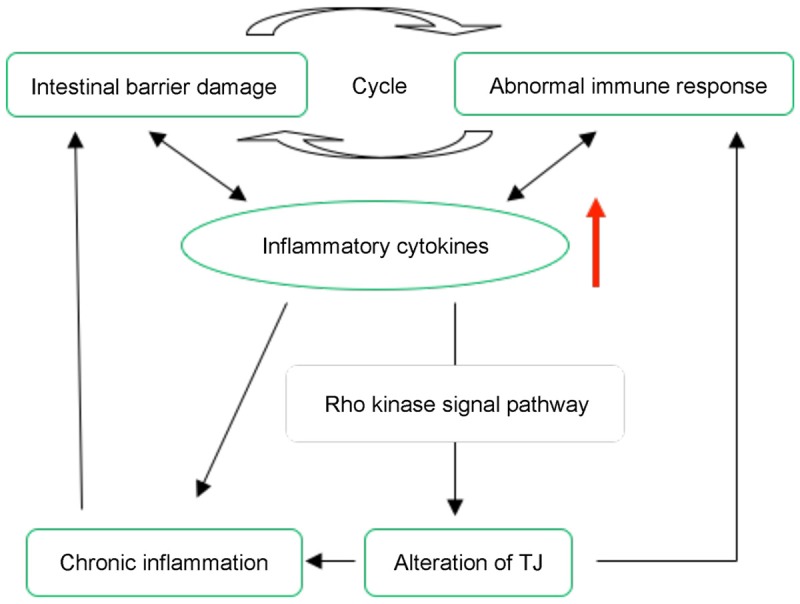
Essential factors for IBD pathogenesis involves Rho signal pathway. Disruptions of the intestinal barrier result in luminal content exposure to mucosal immune cells and activate abnormal immune response, which cause pro-inflammatory cytokines increase. Simultaneously, it can damage the structure of TJ via Rho signal pathway that induced chronic inflammation.
Rho kinase signal pathway in intestinal fibrosis of IBD
Intestinal fibrosis in IBD
In the pathological procedure of IBD, the intestinal mucosa expresses reduplicated damage and recovery with the repeated stimulation of inflammation, which activates intestinal stromal cells that can produce ECM mediated intestinal fibrosis. Histopathological observation indicates that ECM in UC mainly located in rectal mucosa and submucosa, but ECM in CD can be observed in the all intestinal wall [36]. As the present study suggests, the pathogenesis of fibrosis in IBD involves cellular and molecular mechanism [36-39]. The cellular mechanism mainly includes the excessive activation of intestinal stromal cells, fibroblast migration and epithelial mesenchymal transition (EMT). The molecular aspects include the misbalance of ECM; increasing of TGF-β, connective tissue growth factors (CTGFs) [40,41] and other cytokines. Above these mechanisms jointly promote the ECM deposition and lead to intestinal wall fibrosis. If fibrous hyperplasia sustained, the progress will eventually result in intestinal deformation, narrow lumen, and even cause intestinal obstruction and other severe complications [42].
Rho kinase signal pathway in intestinal fibrosis of IBD
Based on the related research about the cellular and molecular mechanism of the fibrosis, it is shown that the Rho kinase signal pathway makes sense (Figure 4). Misbalance of ECM is the direct origin of fibrosis, and matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are the crucial factors to maintain normal metabolism of ECM in cells, and Rho/ROCK-dependent actin-cytoskeleton reorganization influences MMPs and TIMPs [43]. In endoscopic biopsy specimens of CD patients, the level of MMPs and its inhibitor TIMPs’ mRNA increased definitely, the level of MMP-2, MMP-4 and TIMP-1 dramatically increased, and the expression of MMP-1’s, MMP-3’s mRNA are 15 times than normal tissues [44,45]. It is also indicated that the activity of TIMP-1 in myofibroblast of CD patients is increased, which can inhibit the increased activity of MMP and result in misbalance of ECM, leading to fast fibrosis reaction [44]. Rho/ROCK caused by abnormal regulation of MMPs/TIMPs controls ECM imbalance, which plays an important part in the development of IBD intestinal fibrosis. IBD’s intestinal wall inflammation activity showed high expression of TGF-β and receptor in the upstream was the starting factors during the process of ECM’s production. Initiating factors accelerate the GTP-Rho into GDP-Rho transformation, acting on the downstream of the cytoskeleton contraction induced by ROCK activated fibroblasts, and secrete a large number of ECM [46]. Simultaneously, the increased level of TGF-β is associated with the expression of CTGF, jointly promoting fiber with the Rho kinase signal pathway [40]. In the study of radiation-induced enteritis, Bourgier [47] found an obvious increase in the intestinal smooth muscle cells and tissues in the coding Rho/ROCK signaling pathway protein gene expression. Its unique inhibitor Y-27632 can specially inhibit the activation of fibroblast, which may be relevant to nuclear factor-kappa B (NF-κB). But all of these are not definitely observed in IBD in vivo and vitro. So, we may put more emphasis on the Rho signal pathway involved in the mechanism of IBD.
Figure 4.
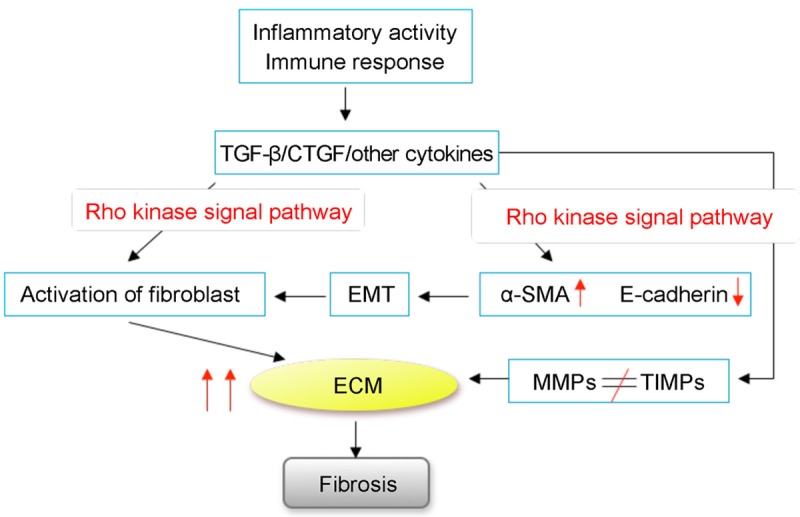
Fibrosis in IBD involves Rho signal pathway. Misbalance of ECM induced by MMPs, TIMPs and activation of fibroblast can be induced by Rho signal pathway. This procedure is activated by the primitive inflammatory response.
Another pathway inducing intestinal fibrosis is EMT, which is considered to be the crucial mechanism of cell transition [48]. Especially, concrete expression can be embodied in: depolymerization of cellular junction; cytoskeleton reorganization; expression of cell adhesion molecules like E-calcium reduced; cell polarity disappeared; mesenchymal markers such as alpha smooth muscle protein (α-SMA), type I collagen enzyme and other proteins expression increased; cell migration. EMT has a pervasive effect on organ’s fibrosis. It has been indicated that epithelial cells of different organs can be changed into myofibroblasts and fibroblasts phenotype which cause the tissue fibrosis, such as alveolar epithelial cells [49] and tubular epithelial cells [50]. Studies on kidney fibrosis suggest that the occurrence of EMT can be also induced by TGF-β [51]. Precisely, in vivo, TGF-β1 secreted by IMF functions as a signal molecule in the upstream increases α-smooth muscle actin (α-SMA), reconfigures actin stress fibers and stimulates actin-myosin contraction in the cell body, finally resulting in EMT. In CD’s patients, the level of TGF-β1, TGF-β2 is apparently elevated in intestinal myofibroblast [52], accordingly, we confer that TGF-β induced EMT in IBD is possibly existed. Identification of EMT as a novel source of fibrosis in the intestinal tract, Flier SN [53] showed that intestinal epithelium detected the feature of mesenchymal cells on the CD model mice induced by TNBS, which prompt EMT happened. At the same time, they found in the intestinal wall sample CD’s patient increased α-SMA expression and E-cadherin expression decreased, but it did not appear in the normal intestinal tissue. As noted above, these can be regarded as EMT’s marker in cell biology. This evidence may support our inference that EMT is a cause of activated fibroblasts and existed in IBD. But for the Rho signal pathway mediated EMT and the occurrence of fibrosis in IBD is still in the exploratory stage, the relevant experimental studies do not illustrate a clear idea of its specific mechanism, which remains to be further exploration and discovery.
Conclusion
Based on the findings described above, it is evident that the Rho kinase signal pathway is involved in the three essential starting segments in the chronic pathogenic procedure of IBD: disruption of the intestinal barrier, exposure of the luminal contents to mucosal immune cells and an abnormal immune response. Furthermore, reduplicate intestinal tract inflammation not only damages the permeability and integrity of intestinal mucosa barrier, but disrupts the balance between MMPs and TIMPs and initiating EMT. The above procedures also can be controlled by Rho kinase signal pathway. Evidence from basic science also revealed that ROCK special inhibitor Y-27632 made sense in improving inflammatory action and fibrosis in experimental model. Thus, studies on the Rho kinase signal pathway should be extremely useful for the precise mechanism of IBD in vivo. In addition, as research moves along, specific inhibition of Rho kinase signal pathway may be a promising novel approach for the treatment of patients with IBD, which effectively functions as preventing inflammatory action, promoting the recovery of intestinal mucosa, reducing fibrosis and improving prognosis.
Disclosure of conflict of interest
None.
References
- 1.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 2.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 3.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 5.Ikeda S, Satoh K, Kikuchi N, Miyata S, Suzuki K, Omura J, Shimizu T, Kobayashi K, Kobayashi K, Fukumoto Y, Sakata Y, Shimokawa H. Crucial role of rho-kinase in pressure overload-induced right ventricular hypertrophy and dysfunction in mice. Arterioscler Thromb Vasc Biol. 2014;34:1260–1271. doi: 10.1161/ATVBAHA.114.303320. [DOI] [PubMed] [Google Scholar]
- 6.Masamune A, Kikuta K, Satoh M, Satoh K, Shimosegawa T. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol. 2003;140:1292–1302. doi: 10.1038/sj.bjp.0705551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duong-Quy S, Bei Y, Liu Z, Dinh-Xuan AT. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. doi: 10.1016/j.pharmthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479–497. doi: 10.1046/j.1440-1746.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 15.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 16.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121:566–579. doi: 10.1053/gast.2001.27060. [DOI] [PubMed] [Google Scholar]
- 18.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 19.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 20.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 22.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 23.Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- 24.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 26.Stallmach A, Giese T, Schmidt C, Ludwig B, Mueller-Molaian I, Meuer SC. Cytokine/chemokine transcript profiles reflect mucosal inflammation in Crohn’s disease. Int J Colorectal Dis. 2004;19:308–315. doi: 10.1007/s00384-003-0554-4. [DOI] [PubMed] [Google Scholar]
- 27.Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–1096. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 30.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Biancheri P, Di Sabatino A, Ammoscato F, Facciotti F, Caprioli F, Curciarello R, Hoque SS, Ghanbari A, Joe-Njoku I, Giuffrida P, Rovedatti L, Geginat J, Corazza GR, MacDonald TT. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol. 2014;44:370–385. doi: 10.1002/eji.201343524. [DOI] [PubMed] [Google Scholar]
- 32.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 34.Segain JP, Raingeard de la Bletiere D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P, Galmiche JP, Loirand G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- 35.Mihaescu A, Santen S, Jeppsson B, Thorlacius H. Rho kinase signalling mediates radiationinduced inflammation and intestinal barrier dysfunction. Br J Surg. 2011;98:124–131. doi: 10.1002/bjs.7279. [DOI] [PubMed] [Google Scholar]
- 36.Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 37.Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20:2198–2206. doi: 10.1097/MIB.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1250–1258. doi: 10.1097/MIB.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beddy D, Mulsow J, Watson RW, Fitzpatrick JM, O’Connell PR. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn’s disease. Br J Surg. 2006;93:1290–1296. doi: 10.1002/bjs.5431. [DOI] [PubMed] [Google Scholar]
- 41.Gunther U, Bateman AC, Beattie RM, Bauer M, MacDonald TT, Kaskas BA. Connective tissue growth factor expression is increased in collagenous colitis and coeliac disease. Histopathology. 2010;57:427–435. doi: 10.1111/j.1365-2559.2010.03652.x. [DOI] [PubMed] [Google Scholar]
- 42.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Schram K, Ganguly R, No EK, Fang X, Thong FS, Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology. 2011;152:2037–2047. doi: 10.1210/en.2010-1166. [DOI] [PubMed] [Google Scholar]
- 44.McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355–1360. doi: 10.1016/S0002-9440(10)63931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 46.Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:226–236. doi: 10.1097/00054725-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Bourgier C, Haydont V, Milliat F, Francois A, Holler V, Lasser P, Bourhis J, Mathe D, Vozenin-Brotons MC. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54:336–343. doi: 10.1136/gut.2004.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoj JP, Davis JA, Fullmer KE, Morrell DJ, Saguibo NE, Schuler JT, Tuttle KJ, Hansen MD. Cellular contractility changes are sufficient to drive epithelial scattering. Exp Cell Res. 2014;326:187–200. doi: 10.1016/j.yexcr.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172–182. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 53.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]


