Abstract
Background: As the most common cardiac arrhythmia, atrial fibrillation (AF) is always accompanied with various complications if without detection and treatment timely. Blood-based pleiotropic molecule biomarkers have now been popularly applied in clinical detection. We hence performed this meta-analysis to evaluate the correlation of serum glycated hemoglobin (HbA1c) levels with the risk of AF in patients with diabetes mellitus (DM). Methods: Covering myriads of computerized databases, we identified potential relevant studies for statistical analysis. We used a standard reporting form to extract data from each included study. Newcastle-Ottawa Scale (NOS) criteria was used for methodological quality assessment. Statistical analyses were conducted with the STATA statistical software. Results: Six cohort studies in full text fulfilled our inclusion criteria, and following overestimation indicated that serum levels of HbA1c in DM patients with AF was higher than that in DM patients without AF (SMD = 0.67, 95% CI: 0.39-0.94, P < 0.001). Subgroup analyses by sample size and detection method implicated that elevated serum HbA1c levels exhibited significant correlations with an increased risk of AF in DM patients in the large-size subgroup (n ≥ 200), the small-size subgroup (n < 200), the high performance liquid chromatography (HPLC) subgroup and the non-HPLC subgroup (Large-size: SMD = 0.70, 95% CI: 0.38-1.03, P < 0.001; Small-size: SMD = 0.64, 95% CI: 0.09-1.19, P = 0.023; HPLC: SMD = 0.81, 95% CI: 0.49-1.12, P < 0.001; Non-HPLC: SMD = 0.36, 95% CI: 0.04-0.68, P = 0.029; respectively). Conclusion: Elevated serum HbA1c levels may be associated with an increased risk of AF in DM patients, possibly reflecting that serum HbA1c level might be a potential biomarker in the prediction of AF in DM patients.
Keywords: Glycated hemoglobin, atrial fibrillation, diabetes mellitus, meta-analysis
Introduction
Atrial fibrillation (AF), which may substantially produce excess cardiovascular prevalence and deaths, has been described as the most frequent continuous arrhythmia, and it can result in left atrial enlargement, upregulated afterload, and increased filling pressures [1,2]. Clinically, the symptoms of AF may be composed of palpitations, fainting, shortness of breath, chest pain, and a decrease in exercise tolerance [3]. As a clinical complication of cardiopulmonary disorders, AF is considered to significantly affect human health and life quality, and the incidence and mortality of AF is estimated to increase in the next few decades due to ageing population [4-6]. According to statistics, the annual incidence of AF in females and males under the age of 65 years is 1.9 per 1000 person-years and 3.1 per 1000 person-years, respectively; and AF is more prevalent in individuals 80 years and older, with the annual prevalence being over 32 per 1000 person-years [7]. Generally, AF seems to develop from the interplay of both environmental factors and molecular mechanism [8,9]. Apart from older age, a variety of other factors involving hypertension, obesity, diabetes, valvular heart disease, ischaemic heart disease, and heart failure may play crucial parts in the risk of new-onset AF [5,9]. There is also evidence showing that serum levels of glycated hemoglobin (HbA1c) may be related with the occurrence of AF [8,10].
HbA1c is considered as the product of a non-enzymatic reaction of glycation, and its amount may be closely linked to concentration of blood glucose [11]. It is widely accepted that HbA1c is an effective marker of long-term regulation of glucose, and it significantly correlates with glycometabolic disease, containing impairment in glucose tolerance, damage in fasting glucose or metabolic syndrome [12,13]. As an advanced glycation end products (AGE), HbA1c could be utilized as a clinical marker of average glycemia in patients with diabetes mellitus (DM) and has been lately advised as an important criterion for the diagnosis of DM [14]. In previous documents, elevated level of HbA1c has been illustrated to be connected with increased risk of cardiovascular diseases in patients with and without DM [15-17]. In addition, it has been demonstrated that HbA1c possesses the ability to reflect antecedent glycometabolic disturbance in myocardial infarction for the fact that it is minimally influenced by stress hyperglycemia during the disease progress [18]. In recent years, serum levels of HbA1c have also been indicated to be correlated with the pathogenesis of AF [10]. Nevertheless, the potential mechanism by which HbA1c may lead to the etiology of AF has remained largely unknown. We postulated that increased levels of HbA1c may lead to high oxygen affinity of hemoglobin and decreased synthesis of diphosphoglycerate, both of which contribute to the difficulty in the dissociation of oxygen dissociation, thereby resulting in aggravated myocardial ischemia and hypoxia [17]. More importantly, long-term high levels of blood glucose in vivo could induce the activation of glycation end products and the up-regulation of tissue growth factor, cause atrial structural remodeling and atrial enlargement, and thus conduce to the development of AF [19]. On the other hand, it was hypothesized that AGEs including HbA1c may play a crucial role in atrial remodeling via the induction of reactive oxygen species (ROS), and may be consequently implicated in the pathogenesis of AF [20]. Up to date, only a few studies have been implemented to investigate the correlation between serum HbA1c levels and the risk of AF in DM patients, and the results are always discordant [21,22]. Hence, we endeavored to determine whether there was a significant connection between serum HbA1c levels and AF risk in DM patients.
Materials and methods
Literature search
The following computerized bibliographic databases were applied to identify relevant articles related to the association of serum HbA1c concentration and AF in DM patients without restrictions with respect to language or data collection: PubMed, Embase, CINAHL, MEDLINE, Science Citation Index database, the Cochrane Library Database, Current Contents Index, Chinese Biomedical Database, the Chinese Journal Full-Text Database and the Weipu Journal Database. (“Atrial Fibrillation” or “Atrial Fibrillation” or “Familial Atrial Fibrillation” or “Auricular Fibrillation”) and (“Hemoglobin A, Glycosylated” or “hemoglobin A1c protein, human” or “Hb A1a+b” or “Hb A1c” or “HbA1” or “Glycosylated Hemoglobin A” or “Glycohemoglobin A” or “Glycosylated A1b Hemoglobin” or “Hb A1b” or “Glycosylated Hemoglobin” or “Glycated Hemoglobins” or “HbA1c” or “hemoglobin A1c”) and (“Diabetes Mellitus” or “diabetes” or “diabetic mellitus” or “DM” or “type 1 diabetes mellitus” or “type 2 diabetes mellitus” or “T2DM” or “T1DM” or “type 1 diabetes” or “type 2 diabetes”) were entered in the databases searches as medical subject heading terms and text words with a highly sensitive search strategy. Manual searches were used to screen other eligible studies.
Study selection
After reading the abstract, full papers were retrieved and assessed for their suitability with the following inclusion criteria: (1) only those cohort studies conducted within a human population to exam the correlations between serum HbA1c levels and the risk of AF in DM patients were incorporated; (2) DM patients with AF should be confirmed by the electrocardiography results [23]; (3) the article must be published in a peer-reviewed journal and provide original data; (4) had supply sufficient information on serum HbA1c levels in DM patients. The major exclusion criteria were: (1) did not satisfy the inclusion criteria designed in the current study; (2) abstracts, reviews, case report, letters, meta-analysis or proceedings; (3) duplication publications or studies with overlapping data.
Data extraction and quality assessment
We used a standard reporting form to extract data from each included study, and the following descriptive information were collected: surname and initials of the first author, year of submission, country, racial descent, study design, number of cases and controls, demographic variables, detection method of HbA1c, serum HbA1c levels and confirmation of diagnosis, etc. Two reviewers independently assessed the methodological quality of the included trials using the Newcastle-Ottawa Scale (NOS) criteria to ensure consistency in reviewing and reporting results [24]. Three aspects were considered in the NOS criteria: (1) subject selection: 0~4; (2) comparability of subject: 0~2; (3) clinical outcome: 0~3. The range of NOS scores is from 0 to 9; and a score ≥ 7 means a good quality. Disagreement on the inclusion of a single study was settled by discussion, or a third investigator was consulted.
Statistical analysis
The association between serum HbA1c levels and the risk of AF in DM patients was estimated by the standardized mean difference (SMDs) and 95% confidence interval (95% CI). We used Cochran’s Q-statistic (P < 0.05 was considered significant) and I2 tests to quantify heterogeneity among studies [25]. In order to calculate the pool SMDs, fixed/random effects model were used; random effects model was applied for the evidence of significant heterogeneity (P < 0.05 or I2 test exhibited > 50%), whereas SMDs were pooled based on the fixed-effects model [25,26]. Meanwhile, if there was significant heterogeneity, subgroup analysis was performed to find potential explanatory variables. In addition, we employed a sensitivity analyses to evaluate whether one single study had the weight to impact on the overall estimate. Further, the effect of publication bias was detected by Egger’s linear regression test (P < 0.05 was considered significant) which can be used to evaluate the funnel plot asymmetry whose asymmetric plot revealed possible publication bias [27,28]. Statistical analyses were conducted with the STATA statistical software (Version 12.0, Stata Corporation, College Station, TX, USA).
Results
Included studies
Figure 1 presented the steps of selecting study. A total of 76 reports were retrieved initially through electronic database searching and manual search, and 31 papers were kept after remove of duplicates (n = 2), letters, reviews or meta-analysis (n = 6), non-human studies (n = 17), and the studies unrelated to research topics (n = 20). Furthermore, additional 23 studies were excluded in that they were not cohort or cohort study (n = 5), not relevant to HbA1c (n = 6), or irrelevant to AF (n = 12). In the final selection step, six out of eight studies were identified with 2 articles being abandoned for not supplying enough information. From 2001 to 2014, the number of articles published in those electronic databases was shown in Figure 2. Six cohort studies in full text hit our selection criteria ultimately between 2008 and 2013 [5,8,21,22,29,30]. Demographic characteristics and methodological quality of the enrolled studies was listed in Table 1. Five studies were performed in populations of Asian descent (China and Turkey), and the remaining 1 study was in populations of Caucasian descent (Italy), including 1,699 subjects at all (1,047 cases and 652 controls). Serum level of HbA1c protein in DM patients were detected with high performance liquid chromatography (HPLC) (n = 4), turbidimetric immunoinhibition assay (n = 1), and colorimetric and immunoturbidimetrical methods (n = 1).
Figure 1.
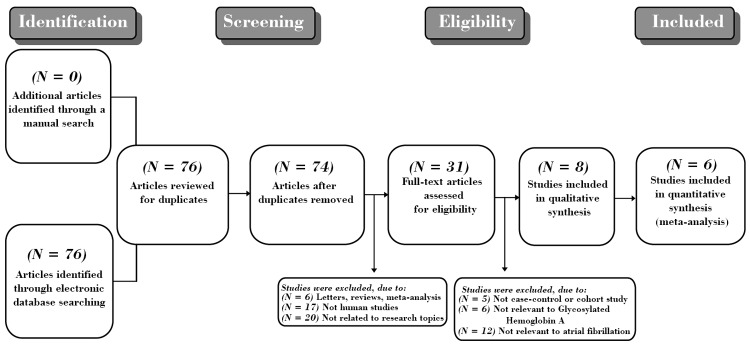
Flow chart of literature search and study selection. Six cohort studies were included in this meta-analysis.
Figure 2.
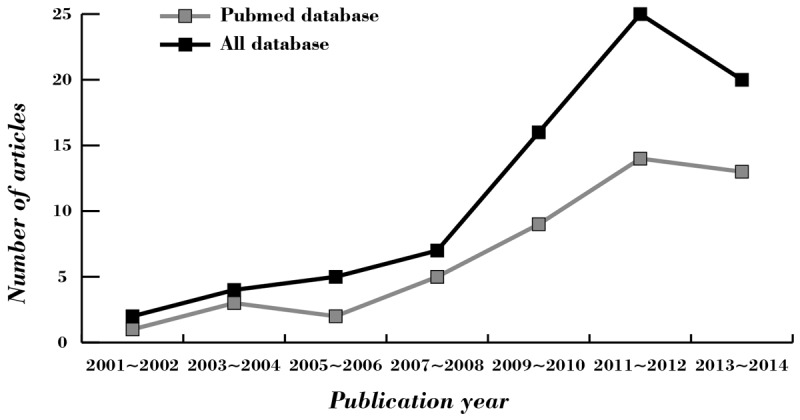
The distribution of the number of topic-related literatures in the electronic database during the last decade.
Table 1.
Baseline characteristics of included studies
| First author | Year | Country | Ethnicity | Sample size | Gender (M/F) | Age (years) | NOS score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Total | AF | Non-AF | AF | Non-AF | AF | Non-AF | |||||
| Xu KL [30] | 2013 | China | Asians | 332 | 180 | 152 | 102/78 | 83/69 | 69.38 ± 4.68 | 69.32 ± 4.63 | 7 |
| Turgut O [21] | 2013 | Turkey | Asians | 162 | 81 | 81 | 84 | 78 | (38~89) | 6 | |
| Targher G [5] | 2013 | Italy | Caucasians | 702 | 617 | 85 | 379 | 323 | 65 ± 13 | 75 ± 9 | 8 |
| Zhao KJ [29] | 2012 | China | Asians | 260 | 83 | 177 | 49/34 | 88/89 | 68.51 ± 8.05 | 64.28 ± 8.34 | 6 |
| Wang XP [22] | 2011 | China | Asians | 89 | 40 | 49 | 18/22 | 24/25 | (36~72) | (35~75) | 5 |
| Johansen OE [8] | 2008 | China | Asians | 154 | 46 | 108 | 34/12 | 73/35 | NR | NR | 5 |
M: male; F: female; NOS: newcastle-ottawa scale; AF: atrial fibrillation; NR: not reported.
Serum levels of HBA1C in AF
As shown in Figure 3, our results revealed that serum level of HbA1c in DM patients with AF was higher than that in DM patients without AF (SMD = 0.67, 95% CI: 0.39-0.94, P < 0.001). Subgroup analyses by sample size and detection method implicated that elevated serum HbA1c levels exhibited significant correlations with an increased risk of AF in DM patients in the large-size subgroup (n ≥ 200), the small-size subgroup (n < 200), the high performance liquid chromatography (HPLC) subgroup and the non-HPLC subgroup (Large-size: SMD = 0.70, 95% CI: 0.38-1.03, P < 0.001; Small-size: SMD = 0.64, 95% CI: 0.09-1.19, P = 0.023; HPLC: SMD = 0.81, 95% CI: 0.49-1.12, P < 0.001; Non-HPLC: SMD = 0.36, 95% CI: 0.04-0.68, P = 0.029; respectively) (as shown in Figure 4).
Figure 3.
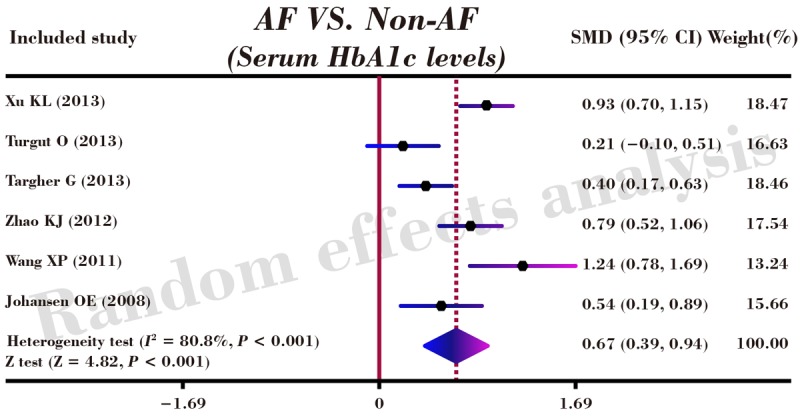
Forest plots for the correlations of serum HbA1c levels with the risk of atrial fibrillation in patients with diabetes mellitus.
Figure 4.
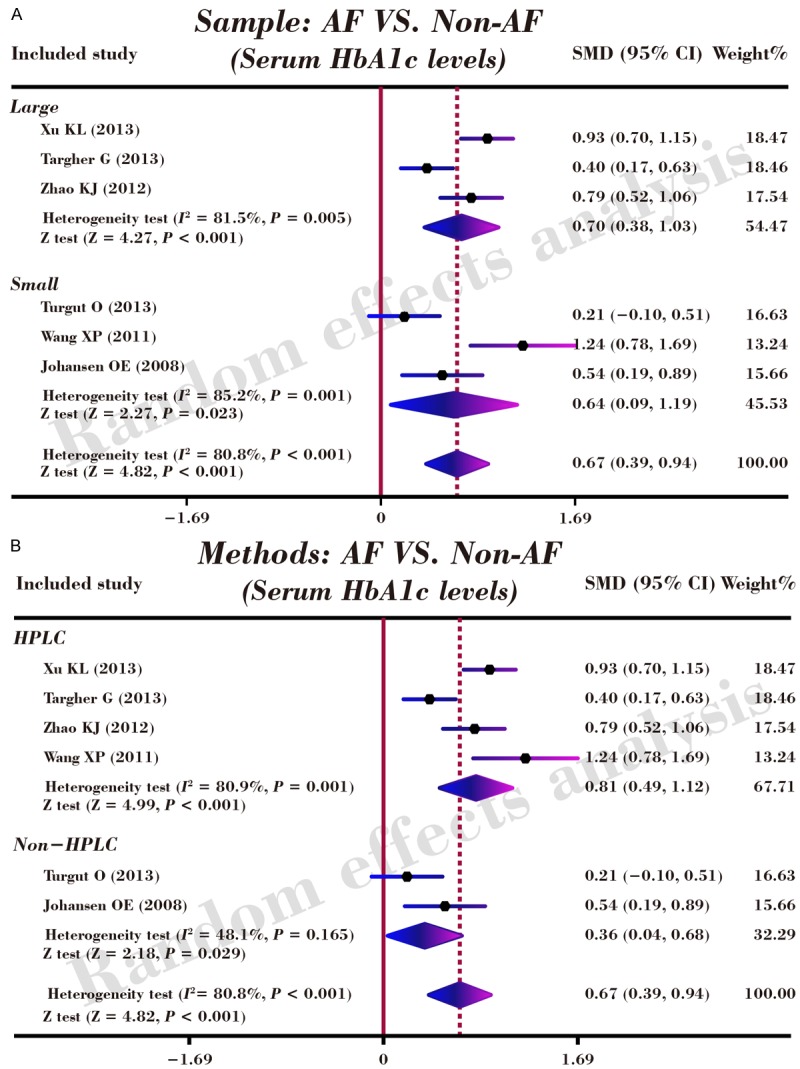
Subgroup analyses by sample size and detection method for the correlations of serum HbA1c levels with the risk of atrial fibrillation in patients with diabetes mellitus.
Sensitivity analysis and publication bias
A leave-one-out sensitivity analysis was carried out to evaluate whether the present meta-analysis is stable. Each study enrolled in our meta-analysis was evaluated one by one to reflect the effect the significance of pooled SMDs. The overall statistical significance does not change when any single study was omitted. Therefore, the current meta-analysis data is relatively stable and credible (Figure 5). The graphical funnel plots of those 6 studies presented to be symmetrical, and Egger’s test suggested no publication bias (t = 0.35, P = 0.747) (Figure 6).
Figure 5.
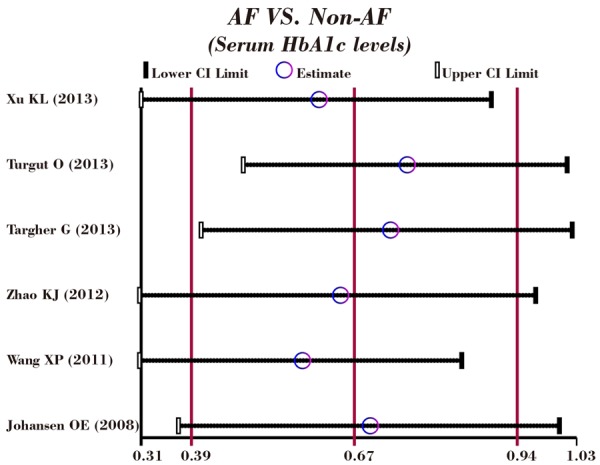
Sensitivity analysis of the summary odds ratio coefficients on the correlations of serum HbA1c levels with the risk of atrial fibrillation in patients with diabetes mellitus.
Figure 6.
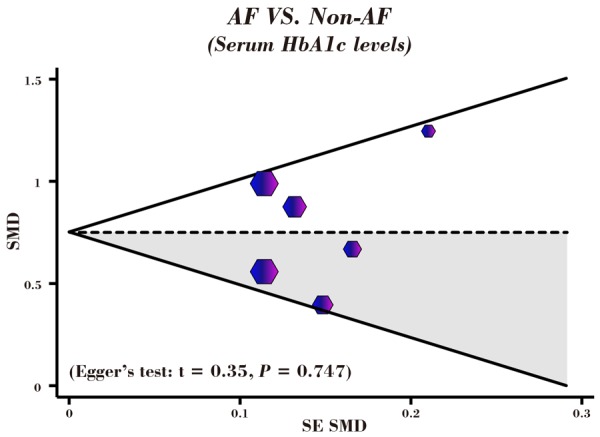
Funnel plot of publication biases on the correlations of serum HbA1c levels with the risk of atrial fibrillation in patients with diabetes mellitus.
Discussion
In this study, we aimed to evaluate the roles of HbA1c and define the relationships of serum HbA1c levels and the risk of AF in DM patients. Findings from the current meta-analysis have shown that higher serum levels of HbA1c triggered the incidence of AF, implying that serum HbA1c level can be viewed as a biologic marker to predict AF morbidity and as a tool for preventing AF onset. HbA1c roles were manifested in multiple aspects, for example, hypercoagulability, dyslipidemia, sematic inflammation and hyperglycemia through accumulating insulin resistance and inducing pro-inflammatory mediators like fibrinogen and C-reactive protein [31]. It was proved that increasing HbA1c levels are responsible for various adverse events such as ventricular damage, electrolyte balance impairment, tissue anoxic phenomenon and systemic inflammation, all of which may lead to up-regulated AF vulnerability, suggesting that HbA1c serum levels are correlated with the occurrence of AF [32]. Moreover, AF incidence has also revealed to be relevant connection with diabetes duration that implicates in an abnormal glucose metabolism, consequently resulting in elevated HbA1c serum levels in turn posing a threat to AF burden [8]. Additionally, AF presence is connected with blood stability according to estimated glomerular filtration rate (eGFR), and HbA1c activities mainly focus on affecting red blood cell to transport oxygen and causing glomerular thickening, meaning that serum levels of HbA1c contribute to increased annual AF incidence [33]. Furthermore, it was reported that the pathogenesis of AF may attribute to the blood coagulation involving in arrhythmia and the role of HbA1c is identified to function in increasing blood viscosity and stimulating blood lipid production, thereby, HbA1c expression may account for AF risk due to its biologic functions [34]. On account of above published opinions, our meta-analysis supported the significant relationship between serum HbA1c levels and the incidence of AF in DM patients, and elevated HbA1c levels might correlated with the deteriorated AF that provoking severe complications, thus serum HbA1c level detection has great recognized value in the diagnosis of AF and can be regarded as therapeutic target for the prevention of AF aggravation. In accordance with our study results, a previous study pointed out that AF development is accountable for various factors including aging, obesity and especially higher HbA1c serum levels, which are explained by the interactions between glycemic control and AF incidence, thus the results indicated that HbA1c levels may be associated with diabetes and AF predisposition [35].
In addition, our study also performed subgroup analysis with the purpose of considering some factors like sample size, detection method to impact the authenticity of the research relationship of HbA1c serum level and AF pathogenesis. A sample-stratified analysis found out that the sample size has no influence in the relationship results, implying that no matter large sample or small sample is not the heterogeneity source for the research. With regard to detection method factor, the results reflected that the relationship cannot be affected by the testing types of method, regardless of HPLC and Non-HPLC. All in all, our study conclusion has shown that AF development is deteriorated along with increasing serum level of HbA1c and the clinical target of AF expression level might be of importance in indicating and treating AF.
Although we have finally came to a conclusion with regard to the relationship between serum HbA1c levels and the development of AF, the relatively smaller involvement of publishes and relatively small proportion of subjects might have the potential to restrict the power of this meta-analysis to conducted our extensive modeling or adjusted analysis. In addition, due to the reason that we focused on the serum levels of HbA1c more frequently, and some well-controlled patients with diabetes who actually held the risk of AF might possibly be grouped into the low serum levels group. Thirdly, our inclusion of literatures covered mostly the parts of the Asian populations and especially among Chinese, remaining population that were not involved should be further investigated. Finally, our exploration results based on previous retrospective might prone to conservative, largely due to those predefined criteria setting for sample inclusion indicated to be relatively narrowly, and some AF patients did not visit hospital regularly, all of which might underestimate of AF.
Taken together, our data suggested that elevated serum HbA1c levels may be associated with an increased risk of AF in DM patients, possibly reflecting that serum HbA1c level might be a potential biomarker in the prediction of AF in DM patients. Nevertheless, data from non-Asian populations were extremely lacking, and further datasets are warranted to investigate the associations of HbA1c with the incidence of AF in DM patients.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–93. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 2.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation. 2010;121:667–74. doi: 10.1161/CIRCULATIONAHA.109.885806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, Van Gelder IC, Ellinor PT, Benjamin EJ. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012;125:2933–43. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Mantovani A, Pichiri I, Rigolon R, Dauriz M, Zoppini G, Morani G, Vassanelli C, Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond) 2013;125:301–9. doi: 10.1042/CS20130036. [DOI] [PubMed] [Google Scholar]
- 6.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–93. doi: 10.1161/CIRCULATIONAHA.110.986661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen OE, Brustad E, Enger S, Tveit A. Prevalence of abnormal glucose metabolism in atrial fibrillation: a case control study in 75-year old subjects. Cardiovasc Diabetol. 2008;7:28. doi: 10.1186/1475-2840-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–95. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Iguchi Y, Kimura K, Shibazaki K, Aoki J, Sakai K, Sakamoto Y, Uemura J, Yamashita S. HbA1c and atrial fibrillation: a cross-sectional study in Japan. Int J Cardiol. 2012;156:156–9. doi: 10.1016/j.ijcard.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Braga F, Dolci A, Mosca A, Panteghini M. Biological variability of glycated hemoglobin. Clin Chim Acta. 2010;411:1606–10. doi: 10.1016/j.cca.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 13.Jesudason DR, Dunstan K, Leong D, Wittert GA. Macrovascular risk and diagnostic criteria for type 2 diabetes: implications for the use of FPG and HbA (1c) for cost-effective screening. Diabetes Care. 2003;26:485–90. doi: 10.2337/diacare.26.2.485. [DOI] [PubMed] [Google Scholar]
- 14.Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327-1334. Clin Biochem Rev. 2009;30:197–200. [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 16.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 17.Rasoul S, Ottervanger JP, Bilo HJ, Timmer JR, van’ t Hof AW, Dambrink JH, Dikkeschei LD, Hoorntje JC, de Boer MJ, Zijlstra F. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med. 2007;65:95–100. [PubMed] [Google Scholar]
- 18.Gustafsson I, Kistorp CN, James MK, Faber JO, Dickstein K, Hildebrandt PR, Group OS. Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J. 2007;154:470–6. doi: 10.1016/j.ahj.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 19.Kato T, Yamashita T, Sekiguchi A, Tsuneda T, Sagara K, Takamura M, Kaneko S, Aizawa T, Fu LT. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol. 2008;19:415–20. doi: 10.1111/j.1540-8167.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong RK, Pettit AI, Quinn PA, Jennings SC, Davies JE, Ng LL. Advanced glycation end products stimulate an enhanced neutrophil respiratory burst mediated through the activation of cytosolic phospholipase A2 and generation of arachidonic Acid. Circulation. 2003;108:1858–64. doi: 10.1161/01.CIR.0000089372.64585.3B. [DOI] [PubMed] [Google Scholar]
- 21.Turgut O, Zorlu A, Kilicli F, Cinar Z, Yucel H, Tandogan I, Dokmetas HS. Atrial fibrillation is associated with increased mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2013;24:493–7. doi: 10.3109/09537104.2012.725876. [DOI] [PubMed] [Google Scholar]
- 22.Wang XP. [Preliminary study on the relationship between type 2 diabetic patients with atrial fibrillation] . Prevention and Treatment of Cardio-Cerebral-Vascular Disease. 2011;11:201–2. [Google Scholar]
- 23.Bollmann A, Husser D, Mainardi L, Lombardi F, Langley P, Murray A, Rieta JJ, Millet J, Olsson SB, Stridh M, Sornmo L. Analysis of surface electrocardiograms in atrial fibrillation: techniques, research, and clinical applications. Europace. 2006;8:911–26. doi: 10.1093/europace/eul113. [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ. 1998;316:471. [PMC free article] [PubMed] [Google Scholar]
- 28.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 29.Zhao KJ, Wu XQ. [Relationship between glycated hemoglobin levels in atrial fibrillation] . Shandong Medical Journal. 2012;52:41–2. [Google Scholar]
- 30.Xu KL, Long T. [Analysis of serum lycated hemoglobin, bilirubin and uric acid concentrations in non-diabetic elderly patients with atrial fibrillation] . Chinese Journal of Laboratory Diagnosis. 2013;17:2183–4. [Google Scholar]
- 31.Hong LF, Li XL, Guo YL, Luo SH, Zhu CG, Qing P, Xu RX, Wu NQ, Li JJ. Glycosylated hemoglobin A1c as a marker predicting the severity of coronary artery disease and early outcome in patients with stable angina. Lipids Health Dis. 2014;13:89. doi: 10.1186/1476-511X-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita T, Asai T, Suzuki T, Kambara A, Matsubayashi K. Preoperative hemoglobin A1c predicts atrial fibrillation after off-pump coronary bypass surgery. Eur J Cardiothorac Surg. 2012;41:102–7. doi: 10.1016/j.ejcts.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iguchi Y, Kimura K, Shibazaki K, Aoki J, Kobayashi K, Sakai K, Sakamoto Y. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol. 2010;106:1129–33. doi: 10.1016/j.amjcard.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Fujii S, Shibazaki K, Kimura K, Sakai K, Aoki J. A simple score for predicting paroxysmal atrial fibrillation in acute ischemic stroke. J Neurol Sci. 2013;328:83–6. doi: 10.1016/j.jns.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu RK, Conen D, Tedrow UB, Fitzgerald KC, Pradhan AD, Ridker PM, Glynn RJ, Albert CM. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. doi: 10.1161/JAHA.114.000916. [DOI] [PMC free article] [PubMed] [Google Scholar]


