Abstract
Purpose: Icariin, a flavonoid isolated from the traditional Chinese herbal medicine Epimedium brevicornum Maxim, has been shown to process anti-inflammatory, antioxidative actions and anti-atherosclerosis activity in vivo and in vitro. The purpose of this study was to investigate the effects and mechanisms of icariin on atherosclerosis by human umbilical vein endothelial cells (HUVECs). Methods: The effects of icariin on the activity of HUVECs induced by oxidized low-density lipoprotein (ox-LDL) were detected by MTT assay. Then we studied the effects of icariin on the adhesion of monocyte with HUVECs induced by ox-LDL. The secretion of E-selectin, intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) by HUVECs were measured by enzyme-linked immunosorbent assay (ELISA) method. Finally the mRNA levels of ICAM-1, VCAM-1, E-selectin of HUVECs were analyzed by real time RT-PCR. Results: MTT result indicated that icariin (10, 20, 40 μmol/L) could inhibit HUVECs injury induced by ox-LDL in a concentration-dependent manner (P < 0.05). The adhesion of monocyte with HUVECs induced by ox-LDL was inhibited by icariin in a concentration-dependent manner (P < 0.05). The levels of ICAM-1, VCAM-1, E-selectin of icariin groups were significantly decreased in a concentration-dependent manner compared with ox-LDL-simulated group (P < 0.05). The mRNA expressions of ICAM-1, VCAM-1, E-selectin of icariin groups were also downregulated significantly compared with ox-LDL-simulated group (P < 0.05). Conclusions: Icariin can prevent atherosclerotic lesion. Its mechanism may be that it can defend against the oxidation damage to HUVECs, inhibit the adhesion of monocyte to HUVECs, and reduce the secretion and expression of adhesion molecules including ICAM-1, VCAM-1, E-selectin.
Keywords: Icariin, atherosclerosis, oxidized low-density lipoprotein, adhesion molecules
Introduction
Atherosclerosis is a chronic vascular inflammatory disease, characterized with the process of lumen narrowing and rigid, as a result of cholesterol and lipid accumulation [1,2]. It is widely accepted that vascular endothelial cells play an important role in initiation and amplification of atherogenesis [3]. Endothelial dysfunction is considered to be a key early stage of atherosclerotic lesions [4]. After endothelial damage, low density lipoproteins enter the subendothelial layer and become oxidized, then the injured endothelial cells synthesize and express various cell adhesion molecules and inflammatory mediator, such as E-selectin, intercellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which contribute to the adhesion and migration of monocytes and the subsequent formation of foam cells [5].
Epimedium brevicornum Maxim, an important traditional Chinese herbal medicine, has been used widely for “tonifying kidney and strengthening bone” for thousands of years in China, Korea, and Japan. Icariin (C33H40O15; molecular weight 676.67; Figure 1), a flavonoid isolated from Epimedium brevicornum Maxim, is considered as the main pharmacological active constituent [6], and has been reported to possess various pharmacological effects, including anti-inflammatory, anti-osteoporosis [7], antitumor [8], immunoregulation [9], and antioxidative actions [10]. In addition, icariin has been shown to have beneficial effects on cardiovascular diseases such as atherosclerosis [11]. However, the potential mechanisms of icariin on atherosclerosis remains incompletely understood. In view of this, the present study was designed to elucidate whether icariin can attenuates the initiation and progression of atherosclerosis. In this study, we want to investigate whether the reduced secretion of cell adhesion molecules is involved in the in vitro protective effects of icariin on the ox-LDL-induced endothelial dysfunction.
Figure 1.
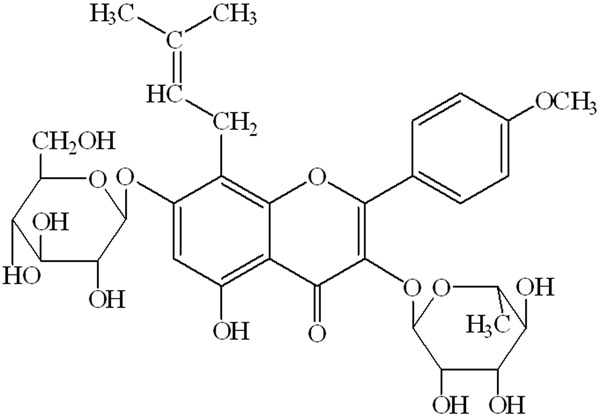
The chemical structure of icariin (C33H40O15; molecular weight = 676.67).
Material and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from American Type Cell Culture (ATCC, Shanghai, China) and cultured in 100 mm dishes in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicilin/streptomycin at 37°C, 5% CO2. THP-1, a human acute monocytic leukemia cell line, was obtained from ATCC and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicilin/streptomycin, and 0.05 mmol/L 2-mercaptoethanol at 37°C, 5% CO2.
Cell viability assay
HUVECs in the logarithmic growth phase were seeded into 96-well plates at a density of 1×104 cells per well, then incubated for 24 hours at 37°C, 5% CO2. After pretreatment with indicated concentration of icariin (purity: > 98%, Vic’s biological technology Co., Ltd, Sichuan, China) (0, 10, 20, 40 μmol/L) for 24 hours, the cells were incubated with or without oxidized low-density lipoprotein (ox-LDL) (Yiyuan Biotechnologies Co., Ltd, Guangzhou, China) (100 μg/mL) for next 24 hours. After suction of the liquid in the wells, MTT solution was added to yield a final concentration of 0.5 mg/mL, and incubation was continued for 4 h at 37°C, 5% CO2. MTT solution was removed gently and 150 μl of dimethyl sulfoxide (DMSO) was added to each well for 15 min incubation. The absorbance of each sample was measrured on a microplate reader at 490 nm as cell viability.
Adhesion of mononuclear cell to HUVECs
HUVECs in the logarithmic growth phase were seeded to 6-well plates for incubation for 24 hours at 37°C, 5% CO2. After pretreatment with indicated concentration of icariin (0, 10, 20, 40 μmol/L) for 24 hours, the cells were incubated with or without ox-LDL (100 μg/mL) for another 24 hours. At the same time, THP-1 cells were labeled with the fluorescent dye BCECF-AM, at a density of 1×106 cells/mL in RPMI 1640 culture medium at 37°C for 1 h. The labeled cells were harvested by centrifugation, washed three times with phosphate-buffered saline (PBS), and the THP-1 cells suspension (150 μl per well) was added to HUVECs growing in 96-well culture plates, following incubation at 37°C for 1 h, and the detached THP-1 cells were removed by stringent washing four times with PBS. The adherent cells were measured through fluorescence intensity at 485 nm and emission wave length at 538 nm using a fluorescence microplate reader.
Secretion of ICAM-1, VCAM-1 and E-selectin
HUVECs in the logarithmic growth phase were seeded to 6-well plates for incubation for 24 hours at 37°C, 5% CO2. After pretreatment with indicated concentration of icariin (0, 10, 20, 40 μmol/L) for 24 hours, the cells were incubated with or without ox-LDL (100 μg/mL) for another 24 hours. ICAM-1, VCAM-1 and E-selectin levels in the cultural supernatants were measured by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. The absorbance of each sample was measrured on a microplate reader at 450 nm.
Total RNA was extracted from HUVECs using Trizol according to the manufacturer’s protocol. RNA was reverse-transcribed with oligo (dT) primers and real-time RT-PCR was conducted with gene-specific primers in the presence of SYBR Premix Ex Taq. qPCR was performed in triplicate, using GAPDH as a the housekeeping control. The real-time RT-PCR amplification was performed with 40 cycles (95°C, 10 s; 60°C, 15 s; 72°C, 30 s) with the following oligonucleotide primer sets (Generay Biotech Co., Ltd, Shanghai, China): ICAM-1 sense: 5’-AGATCATACGGGTTT-GGGCTTC-3’, ICAM-1 antisense: 5’-TATGACTCG-TGAAAGAAATCAGCTC-3’, VCAM-1 sense: 5’-TG-GGCTGTGAATCCCCATCT-3’, VCAM-1 antisense: 5’-GGGTCAGCGCGTGGAATTGGTC-3’, E-selectin sense: 5’-CCATTCGGCCTCTTCAAGCTA-3’, E-se-lectin antisense: 5’-TGCAGCTCACAGAGCCATTC-3’, GAPDH sense: 5’-CATGTTCGTCATGGGTGTG-AACCA-3’, GAPDH antisense: 5’-ATGGCATGGA-CTGTGGTCATGAGT-3’.
The threshold cycle, ΔCt, was calculated as Ct (target gene) - Ct (GAPDH). The relative expression levels in target gene in different treatment groups were calculated with the 2-ΔΔCt method, where ΔΔCt = ΔCt (treatment) - ΔCt (control).
Statistical analysis
Statistical analysis was performed using SPSS17.0 statistical package (SPSS, Chicago, IL). Data were expressed as mean ± SE. One-way analysis of variance (ANOVA) was applied for multiple comparisons and least significant difference test (LSD-t) was applied for intragroup comparison. Values were considered statistically significant when P < 0.05.
Results
Effects of icariin on cell viability
Cell viability was measured in the present study to evaluate whether icariin protect endothelial HUVECs from injuries induced by ox-LDL. As shown in Figure 2, exposure of the cells to ox-LDL for 24 h significantly decreased the cell viability compared with control group (P < 0.05). However, icariin can inhibit cell injury induced by ox-LDL in a concentration-dependent manner, and have significant difference (P < 0.05) compared with ox-LDL-simulated group.
Figure 2.
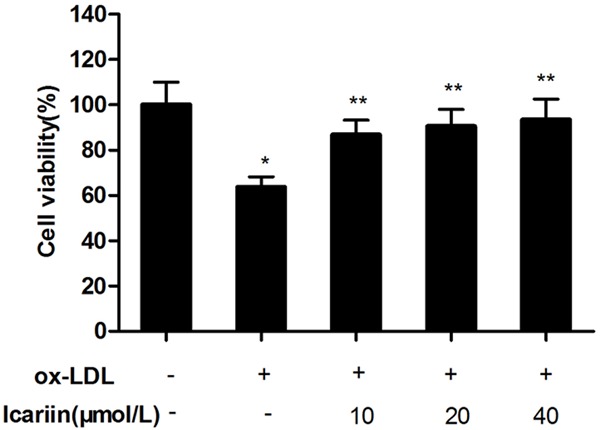
Effects of icariin on ox-LDL induced alteration of cell viability. The HUVECs were pretreated for 24 h with different concentrations of icariin, and then exposed to ox-LDL (100 μg/mL) for another 24 h. Cell viability was assessed by the MTT method. icariin pre-treatment significantly increased the cell viability. *P < 0.05 vs. control group, **P < 0.05 vs. ox-LDL-simulated group.
Effects of icariin on the adhesion of monocytes to HUVECs
Using a fluorescent labelling technique, the effect of icariin on the adhesion of monocytes to HUVECs was measured. As shown in Figure 3, exposure of the cells to ox-LDL for 24 h significantly increased the adhesion of monocytes to HUVECs compared with control group (P < 0.05). However, icariin significantly inhibited cell adhesion caused by ox-LDL, in a concentration-dependent manner compared with ox-LDL-simulated group (P < 0.05).
Figure 3.
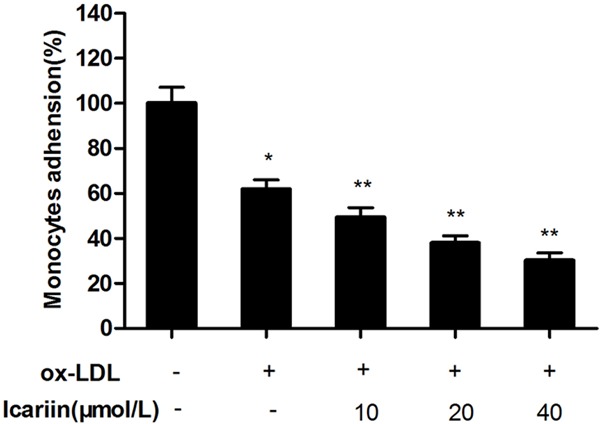
Effects of icariin on the adhesion of monocytes to HUVECs. The HUVECs were pretreated for 24 h with different concentrations of icariin, and then exposed to ox-LDL (100 μg/mL) for another 24 h. The adhesion of monocytes to HUVECs were measured by a fluorescent labelling technique. Icariin pre-treatment significantly inhibited cell adhesion caused by ox-LDL. *P < 0.05 vs. control group, **P < 0.05 vs. ox-LDL-simulated group.
Effects of icariin on secretion of ICAM-1, VCAM-1, E-selectin
As shown in Figure 4, exposure of the cells to ox-LDL for 24 h significantly increased the content of ICAM-1, VCAM-1, E-selectin in culture fluid compared with control group (P < 0.05). However, icariin significantly reduced the secretion of ICAM-1, VCAM-1, E-selectin caused by ox-LDL in a concentration-dependent manner compared with the ox-LDL-simulated group (P < 0.05).
Figure 4.
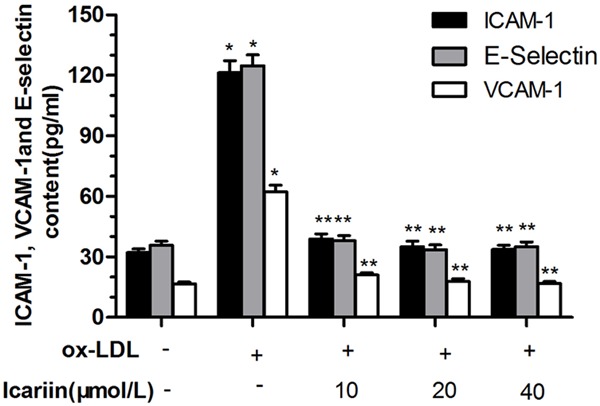
Effects of icariin on secretion of ICAM-1, VCAM-1 and E-selectin. The HUVECs were pretreated for 24 h with different concentrations of icariin, and then exposed to ox-LDL (100 μg/mL) for another 24 h. ICAM-1, VCAM-1 and E-selectin content was evaluated by the ELISA method, which quantified the content as the optical density at 450 nm. *P < 0.05 vs. control group, **P < 0.05 vs. ox-LDL-simulated group.
The RT-PCR result is as shown in Figure 5, ox-LDL induced a significant increase in expression of ICAM-1, VCAM-1, E-selectin compared with control group (P < 0.05). However, icariin significantly reduced the expression of ICAM-1, VCAM-1, E-selectin, and have significant difference (P < 0.05) compared with the ox-LDL-simulated group.
Figure 5.
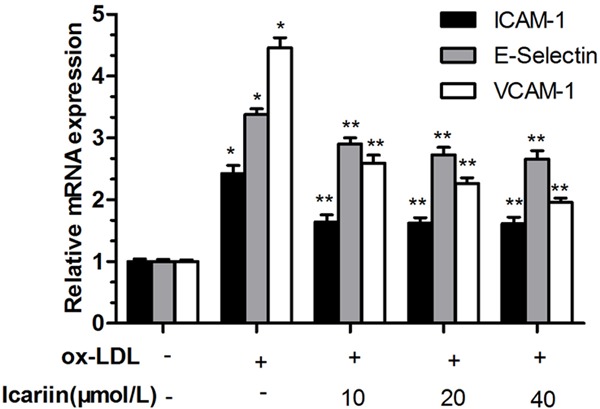
Effects of icariin on expression of ICAM-1, VCAM-1 and E-selectin. *P < 0.05 vs. control group, **P < 0.05 vs. ox-LDL-simulated group.
Discussion
Atherosclerosis is a chronic vascular inflammatory disease [12]. It is characterized by the formation of atherosclerotic plaques consisting of foam cells, leukocytes, platelets, inflamed smooth muscle cells and endothelial cells (ECs) [13]. It is increasingly recognized that ox-LDL play a critical role in the initiation of atherosclerotic lesion formation [14]. Ox-LDL can impair endothelial function, induce the ECs shrinkage, upregulate expression of adhesion molecules on ECs, recruit monocytes to the subendothelial space, change the secretory activities of endothelium, reduce the antioxidant capability, and enhance ECs apoptosis [3]. In consequence, injury of ECs is a critical event in the development of atherosclerosis [15].
ECs at rest do not interact with adhesion molecules and cytokines, however when treated by ox-LDL, ECs upregulate the expression of cell adhesion molecules such as VCAM-1 and ICAM-1, E-selectin, P-selectin [16], which in turn mediates monocytes and lymphocytes recruitment and adherence to the ECs [17]. At the same time, ECs upregulate the expression of various pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-1 (IL-1), which are important mediators of atherosclerosis [18].
In this study, HUVECs were incubated with ox-LDL, the cell viability was decreased and the adhesion to mononuclear cell was increased significantly, icariin can inhibit cell injury and adhesion made by ox-LDL in a concentration-dependent manner. It showed icariin can attenuate the impairment induced by ox-LDL, reduce the adhesion ability of HUVECs to inflammatory cells. At the same time, icariin decrease the secretion and expression of ICAM-1, VCAM-1 and E-selectin activated by ox-LDL, which are important adhesion molecules. ICAM-1, VCAM-1, E-selectin are all best characterized in leukocyte trafficking and monocyte recruitment during the biogenesis and formation of atherosclerotic lesions. E-selectin is thought to mediate monocytes to attach to and to roll along the activated vascular endothelium, and ICAM-1, VCAM-1 are believed to be associated with adhesion and transendothelial migration of monocytes [19].
In summary, our results demonstrate that Icariin exhibit a protective effect on HUVECs injury induced by ox-LDL, and the exact mechanism is associated with anti-adhesion and anti-inflammation. The present data raise the possibility that icariin can reduce the development of atherosclerosis.
Acknowledgements
The present study was financially supported by the grants from Foundation of the Jilin Science and Technology Agency of China (140101JC010217669).
Disclosure of conflict of interest
None.
References
- 1.Dell’omo G, Penno G, Pucci L, Lucchesi D, Fotino C, Del Prato S, Pedrinelli R. ACE gene insertion/deletion polymorphism modulates capillary permeability in hypertension. Clin Sci (Lond) 2006;111:357–364. doi: 10.1042/CS20060165. [DOI] [PubMed] [Google Scholar]
- 2.Zhu F, Li C, Jin XP, Weng SX, Fan LL, Zheng Z, Li WL, Wang F, Wang WF, Hu XF, Lv CL, Liu P. Celastrol may have an anti-atherosclerosis effect in a rabbit experimental carotid atherosclerosis model. Int J Clin Exp Med. 2014;7:1684–1691. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Ren X, Yang Z, Zhao Y, Ji L, Li J, Yue W. Effects and mechanisms of indol-2,3-dione on atherosclerosis. Int J Clin Exp Med. 2014;7:2087–2091. [PMC free article] [PubMed] [Google Scholar]
- 4.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 5.Zhou CH, Pan J, Huang H, Zhu Y, Zhang M, Liu L, Wu Y. Salusin-beta, but not salusin-alpha, promotes human umbilical vein endothelial cell inflammation via the p38 MAPK/JNK-NF-kappaB pathway. PLoS One. 2014;9:e107555. doi: 10.1371/journal.pone.0107555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao-Hong D, Chang-Qin X, Jian-Hua H, Wen-Jiang Z, Bing S. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharm Biol. 2013;51:433–440. doi: 10.3109/13880209.2012.738332. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G, Qin L, Sheng H, Yeung KW, Yeung HY, Cheung WH, Griffith J, Chan CW, Lee KM, Leung KS. Epimedium-derived phytoestrogen exert beneficial effect on preventing steroid-associated osteonecrosis in rabbits with inhibition of both thrombosis and lipid-deposition. Bone. 2007;40:685–692. doi: 10.1016/j.bone.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang DC, Liu JL, Ding YB, Xia JG, Chen GY. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-kappaB. Acta Pharmacol Sin. 2013;34:301–308. doi: 10.1038/aps.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap SP, Shen P, Li J, Lee LS, Yong EL. Molecular and pharmacodynamic properties of estrogenic extracts from the traditional Chinese medicinal herb, Epimedium. J Ethnopharmacol. 2007;113:218–224. doi: 10.1016/j.jep.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Huang JH, Ning Y, Shen ZY. [Icariin and its pharmaceutical efficacy: research progress of molecular mechanism] . Zhong Xi Yi Jie He Xue Bao. 2011;9:1179–1184. doi: 10.3736/jcim20111104. [DOI] [PubMed] [Google Scholar]
- 11.Xu HB, Huang ZQ. Vasorelaxant effects of icariin on isolated canine coronary artery. J Cardiovasc Pharmacol. 2007;49:207–213. doi: 10.1097/FJC.0b013e3180325abe. [DOI] [PubMed] [Google Scholar]
- 12.Nurgeldiyeva MJ, Hojakuliyev BG, Muhammedov MB. Correlation of atherogenesis with an infection of Candida albicans. Int J Clin Exp Med. 2014;7:2137–2143. [PMC free article] [PubMed] [Google Scholar]
- 13.Butcher MJ, Herre M, Ley K, Galkina E. Flow cytometry analysis of immune cells within murine aortas. J Vis Exp. 2011:e2848. doi: 10.3791/2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–347. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Yang X, Peng Y, Lin J. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc Drugs Ther. 2009;23:439–448. doi: 10.1007/s10557-009-6206-3. [DOI] [PubMed] [Google Scholar]
- 16.Bai YP, Hu CP, Chen MF, Xu KP, Tan GS, Shi RZ, Li YJ, Zhang GG. Inhibitory effect of reinioside C on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via inhibiting NADPH oxidase/ROS/NF-kappaB pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:399–406. doi: 10.1007/s00210-009-0450-8. [DOI] [PubMed] [Google Scholar]
- 17.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 18.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takei A, Huang Y, Lopes-Virella MF. Expression of adhesion molecules by human endothelial cells exposed to oxidized low density lipoprotein. Influences of degree of oxidation and location of oxidized LDL. Atherosclerosis. 2001;154:79–86. doi: 10.1016/s0021-9150(00)00465-2. [DOI] [PubMed] [Google Scholar]


