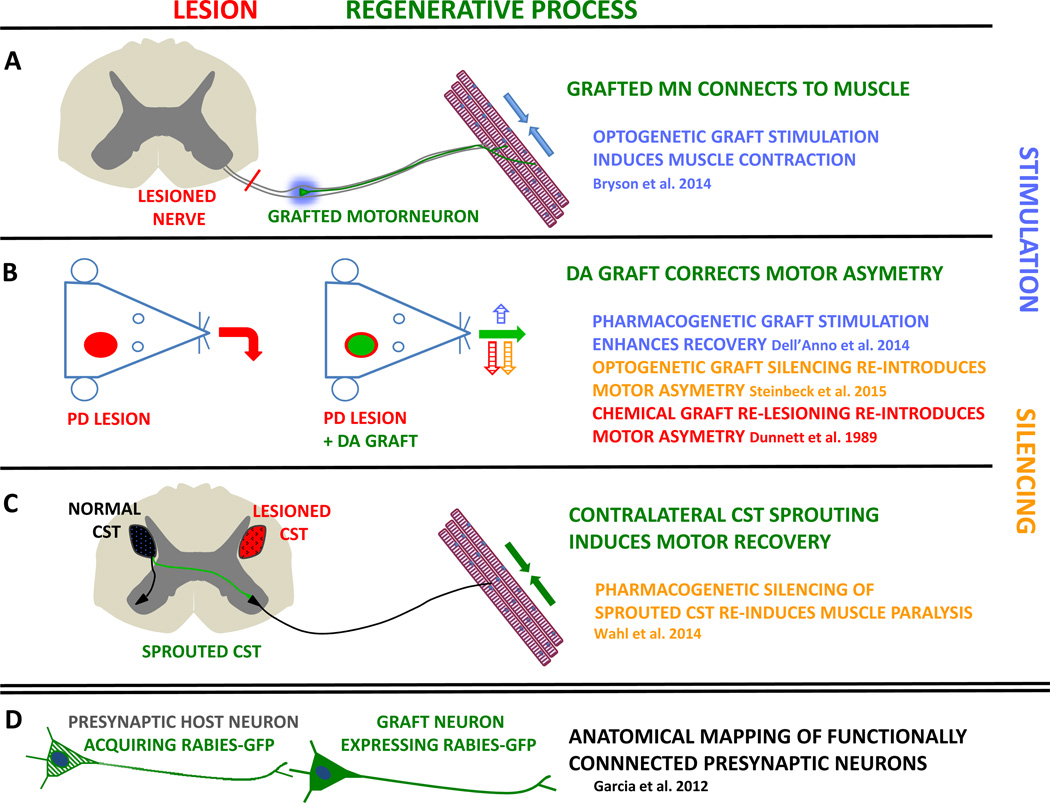
Figure 3. Strategies to assess neuronal graft-to-host connectivity.
For functional experiments optogenetic or pharmacogenetic strategies have been used to stimulate (blue) or silence (orange) donor cells after recovery in behavioral experiments. The initial lesion is depicted in red and the regenerative mechanism in green. A, Spinal motoneurons were injected into the crushed sciatic nerve of mice and donor axons reconnected to limb muscles. Blue light optogenetic stimulation of the graft induced muscle contractions in the leg. B, in a Parkinson’s disease (PD) model with unilateral lesions, dopamine (DA) grafts correct the movement asymmetry. Pharmacogenetic graft stimulation enhances recovery whereas optogenetic graft silencing re-introduces the previous deficit, mimicking the effect chemical re-lesioning. C, rats with unilateral cortico-spinal tract (CST) lesions were subjected to a combination therapy to enhance axonal sprouting (green) from the contralateral CST. This therapy induced motor recovery in the impaired paw. Pharmacogenetic silencing of the new connection resulted in muscle paralysis, demonstrating that axonal sprouting from the healthy CST drives recovery. D, grafted neurons infected with a GFP-tagged rabies virus transfer rabies-GFP (green) to presynaptic host neurons in vivo, thereby revealing the upstream functional connectome of the graft.