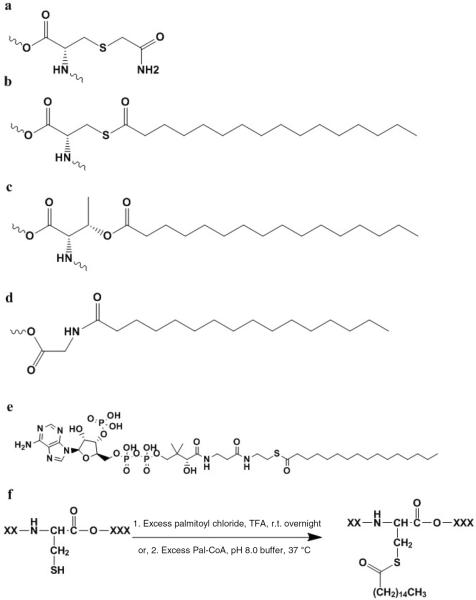
Figure 1.
Sample molecular structures and reaction schematics for the carboxyamidomethylation (Am) or palmitoylation (Pal) modifications. (a) Carboxyamidomethylated cysteine residue (CysAm). (b) Palmitoylated cysteine residue (CysPal), with the modification on the thiol group. (c) Palmitoylated threonine residue (ThrPal), with the modification on the hydroxyl group. (d) N-palmitoylated glycine residue (GlyPal), with the modification on the N-terminus of a peptide or protein. (e) Palmitoyl coenzyme A (Pal-CoA). (f) Palmitoylation reaction schematics. (f-1) Reaction via palmitoyl chloride in TFA solution. (f-2) Reaction via Pal-CoA in pH 8.0 phosphate buffer.