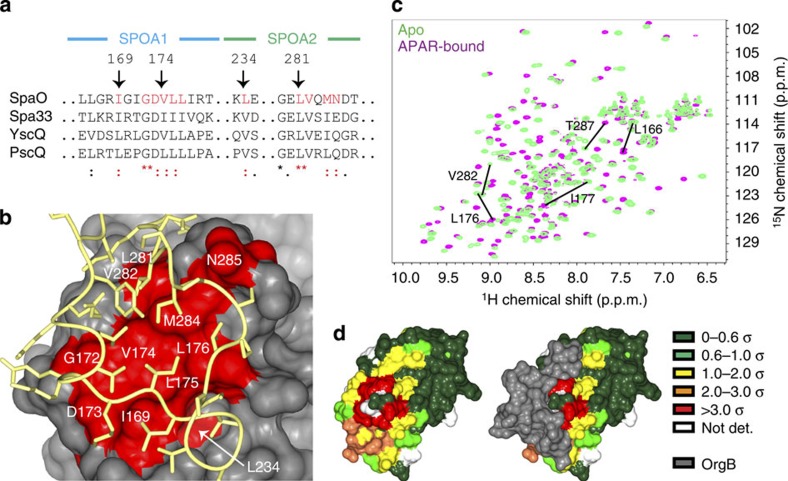
Figure 4. The APAR-binding site of SpaO.
(a) The SpaO residues at the APAR interaction site are highly conserved across homologues in other species. Excerpts of the M-COFFEE alignment of SpaO, Shigella flexneri Spa33, Yersinia enterocolica YscQ and Pseudomonas aeruginosa PscQ are shown with conserved APAR-interacting residues highlighted in red. Symbols beneath the alignment indicate the degree of conservation: asterisks denote full conservation, colons denote strong similarity, and dots denote weak similarity. (b) A surface representation of SpaO with the conserved interfacial residues identified in a are coloured red and the OrgB APAR backbone is yellow. (c) Overlayed 15N-heteronuclear single quantum coherence spectra of apo- (green) and APAR-bound (violet) SpaO(140–297). The five largest peak shifts are noted. (d) The solution interaction data from c are mapped onto the SpaO–OrgB crystal structure. Surface residues are colour coded by the size of their weighted CSD in units of s.d. Residues not assigned an amide resonance in one of the two data sets are left white. The same view of SpaO is shown with and without OrgB (grey surface).