Abstract
Differentiation between endometrial stromal sarcomas (ESSs) and smooth muscle tumors of the uterus can be challenging. Transgelin, a 22 kDa actin-binding protein has recently been shown to be a smooth muscle specific marker. The goal of this study was to determine whether transgelin could accurately distinguish ESSs from smooth muscle tumors.
The expression of transgelin, CD10 and smooth muscle actin (SMA) in 13 ESSs (4 low grade, 6 undifferentiated and 3 metastatic), 9 smooth muscle tumors (1 leiomyoma and 8 leiomyosarcomas (LMSs) and 15 soft tissue LMSs was studied. The diagnostic performance of transgelin compared to the other smooth muscle markers was assessed.
Transgelin was diffusely strongly positive in all myometria, leiomyoma, and uterine and soft tissue LMSs. In contrast, transgelin expression was totally absent in all endometria, primary and metastatic ESSs. SMA positivity was noticed in 4 of the 13 ESSs. CD10 was positive in most ESSs.
Transgelin appears to be a specific marker of smooth muscle differentiation in the uterus with 100% sensitivity and specificity and may be useful for distinguishing LMS from ESS. It could be used as an additional marker useful for decision making, especially in those tumors with questionable histology.
Keywords: Transgelin, endometrial stromal sarcoma, leiomyosarcoma
I. Introduction
Uterine sarcomas are rare mesenchymal neoplasms that comprise about 7% of all soft tissue tumors and up to 3% of uterine malignancies.1,2 Excluding carcinosarcomas (Malignant Mixed Müllerian Tumors), endometrial stromal sarcoma (ESS) and leiomyosarcoma (LMS) represent the majority of this group of tumors.2,3 Traditionally ESS has been categorized into low and high grade tumors based on mitotic activity and the morphologic resemblance of the tumor to endometrial stroma.3-5 Currently, the World Health Organization (WHO) classifies these tumors into low grade and undifferentiated sarcomas.3 Low grade ESSs are composed of neoplastic cells that still resemble the normal, benign proliferative endometrium but with definite evidence of myometrial invasion in the characteristic “finger-like” infiltrative pattern.6,7 They are also known to frequently have lympho-vascular invasion.6,7 In contrast undifferentiated ESSs lack evidence of endometrial stromal differentiation and are clinically more aggressive.3 Recent studies have shown that some of the undifferentiated ESSs have an immunohistochemical and molecular profile that overlaps with that of low grade ESS.8-10 The authors of these studies argue the need for the reclassification of ESS into the current low grade ESS and the splitting of undifferentiated ESS into high grade ESS because of evidence of lower grade component in the tumor and the truly undifferentiated ESS.
The morphologic distinction between ESS and LMS is not straightforward and at times has been shown to be challenging with poor reproducibility. The use of immunohistochemistry with a battery of markers including smooth muscle actin (SMA), desmin, actin, h-caldesmon and CD10 have been proposed to be of value.10-15 However, the current immunohistochemical (IHC) panel has been shown to be not entirely specific and less helpful in this regard.12-14,16,17 Transgelin, a 22 kDa actin-binding protein of the calponin family is a novel marker that recently has been shown to correlate with smooth muscle differentiation.18-21 The promoter of the gene is the target of the transcriptional activator serum response factor of which myocardin acts as a cofactor.21 By using gene expression profiling, studies have shown that transgelin was one of the most promising markers for the leiomyosarcomatous differentiation. A recent gene expression signature study demonstrated an overexpression of several genes including transgelin in LMS as compared to ESS confirming molecular differences between uterine ESS and LMS.22
The goal of this study was to determine if transgelin, a smooth muscle-specific marker, could accurately distinguish ESS from uterine smooth muscle tumors and LMS from other body sites.
II. Methods
This retrospective study was approved by the institutional review committee at the University of Kansas Medical Center. A total of 37 patients diagnosed between 2002 and 2012 were studied. These are composed of 13 ESSs, 1 uterine leiomyoma, 8 uterine LMSs and 15 extra uterine, soft tissue LMSs. All tumors were graded using the WHO grading system.
At diagnosis, tissue blocks containing the most representative and well-preserved tumor areas were selected for IHC analysis. Immunohistochemistry was performed on tissue fixed with 10% neutral buffered formalin. IHC analyses for transgelin (Anti-SM22 alpha antibody (ab14106); pre-treatment: citrate antigen retrieval in the Biocare pressure cooker; dilution: 1:3000; source: abcam, Cambridge, Massachusetts), CD10 (clone 56C6; pre-treatment: CC1, prediluted; source: Cell Marque, Rocklin, California) and SMA (clone: 1A4; pre-treatment: CC1; prediluted; source: Cell Marque, Rocklin, California) were performed.
Positive IHC reactions were defined as dark brown reaction positive cytoplasmic staining for transgelin and SMA and dark brown reaction on the cell membrane for CD10 in at least 5% of the marked lesional cells. Positivity was further divided into focal (<50% cells labeled) and diffuse (>50% cells labeled) patterns. The diagnostic performance of transgelin was assessed in terms of sensitivity, specificity and accuracy. It was first evaluated for the diagnosis of LMS and then was compared with ESS. We did not calculate P-value in case of diagnostic performance because of the superiority of transgelin over the other markers in differentiating LMS from ESS.
III. Results
A total of 37 patients diagnosed between 2002 and 2012 were studied. These are composed of 13 ESSs, 1 uterine leiomyoma, 8 uterine LMSs and 15 extra uterine, soft tissue LMSs. The ESS group included 4 low-grade ESSs, 6 undifferentiated ESSs and 3 metastatic tumors; one to kidney, one to colon and one to lung. The mean age of the ESS patients was 59.8 years (range 25 to 86), the age of the uterine leiomyoma patient was 42, the mean age of the uterine LMS patients was 56.1 (range 29 to 75) and the mean age of the non-GYN soft tissue LMS patients was 62.3 (range 38 to 91).
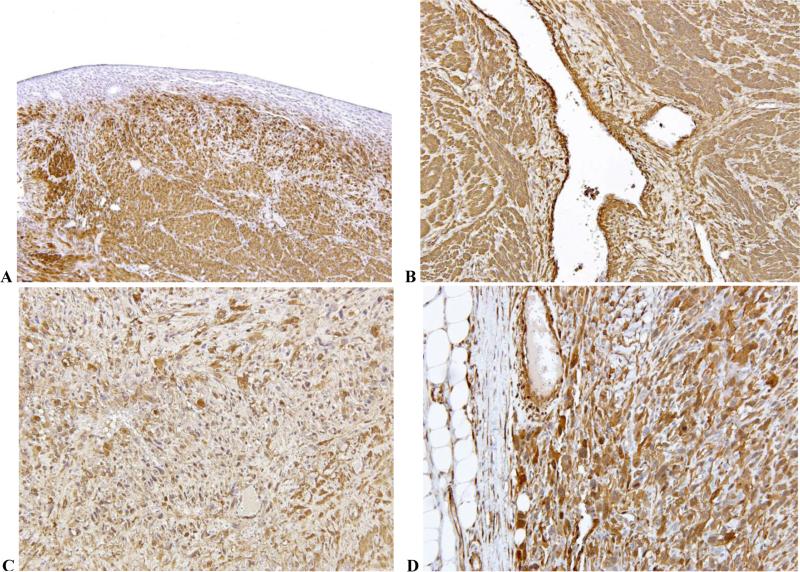
Tables 1 and 2 show patterns of positivity for transgelin, CD10 and SMA in the gynecologic/uterine (GYN) and non-GYN, soft tissue smooth muscle tumors. Transgelin was the most consistently expressed marker (positive in 100% of cases). Smooth muscle actin was also expressed in most of the tumors studied for this marker but with variable intensity between tumors. Fig. 1 shows positive staining pattern for transgelin in normal myometrium (Fig. 1A), leiomyoma (Fig. 1B), GYN LMS (Fig. 1C) and non-GYN LMS (Fig. 1D). There was no difference in staining intensity between normal myometrium and benign or malignant smooth muscle tumors. Also there was no difference in staining intensity between GYN and non-GYN LMSs.
Table 1.
Patterns of positivity for transgelin, CD10 and SMA in gynecologic/uterine smooth muscle tumors
| Case | Diagnosis | Specimen | Age | Transgelin | CD10 | SMA# |
|---|---|---|---|---|---|---|
| 1 | Leiomyoma - Gyn@ | Hysterectomy | 42 | + | ND** | + |
| 2 | LMS* - Gyn | Hysterectomy | 42 | + | - | + |
| 3 | LMS - Gyn | Hysterectomy | 75 | + | + | + focal |
| 4 | LMS - Gyn | Hysterectomy | 75 | + | ND | + |
| 5 | LMS - Gyn | Hysterectomy | 29 | + | ND | ND |
| 6 | LMS - Gyn | Hysterectomy | 39 | + | ND | + |
| 7 | LMS - Gyn | Hysterectomy | 75 | + | ND | + |
| 8 | LMS - Gyn | Hysterectomy | 58 | + | - | + |
| 9 | LMS - Gyn, metastatic | Retrocecal | 56 | + | ND | ND |
LMS: leiomyosarcoma
Gyn: gynecologic, uterine smooth muscle tumor
SMA: smooth muscle actin
ND: not done
Table 2.
Patterns of positivity for transgelin, CD10 and SMA in non-gynecologic, soft tissue leiomyosarcomas
| Case | Diagnosis | Specimen | Age | Transgelin | CD10 | SMA# |
|---|---|---|---|---|---|---|
| 1 | LMS* - non Gyn@ | Calf | 67 | + | ND** | |
| 2 | LMS - non Gyn | Nephrectomy | 42 | + | - | + |
| 3 | LMS - non Gyn | Abdominal | 57 | + | ND | + |
| 4 | LMS - non Gyn | Thigh | 65 | + | ND | + |
| 5 | LMS - non Gyn | Perirectal | 91 | + | ND | + |
| 6 | LMS - non Gyn | Leg | 64 | + | ND | + |
| 7 | LMS - non Gyn | Leg | 81 | + | ND | ND |
| 8 | LMS - non Gyn | Back | 64 | + | ND | ND |
| 9 | LMS - non Gyn | IVC | 38 | + | ND | + |
| 10 | LMS - non Gyn | Thigh | 58 | + | ND | + |
| 11 | LMS - non Gyn, metastatic | Paraspinal | 52 | + | ND | ND |
| 12 | LMS - non Gyn, metastatic | Shoulder | 64 | + | ND | ND |
| 13 | LMS - non Gyn, metastatic | Lung | 82 | + | ND | ND |
| 14 | LMS - non Gyn, metastatic | Lung | 50 | + | ND | ND |
| 15 | LMS - non Gyn, metastatic | Lung | 59 | + | ND | + |
LMS: leiomyosarcoma
non Gyn: non-gynecologic, soft tissue leiomyosarcoma
SMA: smooth muscle actin
ND: not done
Fig. 1.
Composite photomicrograph showing positive transgelin expression in normal uterus (Fig. 1A), uterine leiomyoma (Fig. 1B), uterine leiomyosarcoma (Fig. 1C) and soft tissue leiomyosarcoma (Fig. 1D). [Immunostain, magnification 200X]
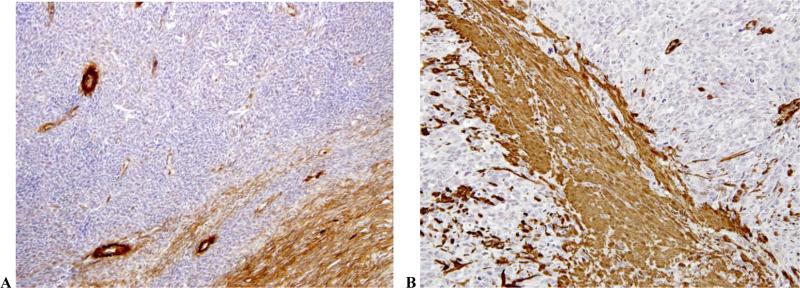
Table 3 shows patterns of positivity for Transgelin, CD10 and SMA in the ESSs. Contrary to smooth muscle tumors, transgelin was consistently not expressed in any of the ESSs studied. As expected, CD10 was expressed in most of the ESSs. Cases with absent or decreased CD10 expression belonged to the undifferentiated ESS group (2 of 6 cases). SMA was noted in 4 of the 13 ESSs studied. Figures 1A, 2A and 2B show lack of transgelin expression in normal endometria (Fig. 1A), low grade ESS (Fig. 2A) and undifferentiated ESSs (Fig. 2B).
Table 3.
Patterns of positivity for transgelin, CD10 and SMA in endometrial stromal sarcomas
| Case | Diagnosis | Specimen | Age | Transgelin | CD10 | SMA# |
|---|---|---|---|---|---|---|
| 1 | ESS*, low grade | Hysterectomy | 49 | - | ND** | ND |
| 2 | ESS, low grade | Hysterectomy | 44 | - | + | - |
| 3 | ESS, low grade | Hysterectomy | 44 | - | + | - |
| 4 | ESS, low grade | Hysterectomy | 51 | - | + | + |
| 5 | ESS, undifferentiated | Hysterectomy | 61 | - | + | - |
| 6 | ESS, undifferentiated | Hysterectomy | 86 | - | + | + focal |
| 7 | ESS, undifferentiated | Hysterectomy | 25 | - | - | - |
| 8 | ESS, undifferentiated | Hysterectomy | 77 | - | + focal | + focal |
| 9 | ESS, undifferentiated | Biopsy | 68 | - | + | + focal |
| 10 | ESS, undifferentiated | Hysterectomy | 65 | - | + | - |
| 11 | ESS, metastatic | Colectomy | 79 | - | ND | ND |
| 12 | ESS, metastatic | Lung | 64 | - | ND | ND |
| 13 | ESS, metastatic | Nephrectomy | 64 | - | + | ND |
ESS: endometrial stromal sarcoma
SMA: smooth muscle actin
ND: not done
Fig. 2.
Photomicrographs showing lack of transgelin expression in low grade (Fig. 2A) and undifferentiated endometrial stromal sarcomas (Fig. 2B). [Immunostain, magnification 200X]
IV. Discussion
The primary goal of this study was to evaluate the diagnostic accuracy of transgelin in effectively distinguishing ESSs from uterine smooth muscle tumors. Transgelin appears to be a specific marker of smooth muscle differentiation in the uterus and other non-GYN sites with 100% sensitivity and specificity.18-22 It could be a useful marker for decision making, especially in those tumors with questionable histology and immunophenotype. Transgelin was consistently overexpressed in normal myometrium and all smooth muscle tumors (leiomyoma and LMS) studied. All ESSs studied were negative for transgelin or with less than 5% weak expression. SMA positivity was noticed in 4 of the 13 ESSs. Of interest, none of the smooth muscle tumors expressed 100% positivity with any of the smooth muscle markers at any given time, as was the case with transgelin.
Although the morphologic distinction between ESS and LMS is straightforward in many cases, at times tumor classification has been shown to be challenging with poor reproducibility. On one end of the spectrum, differentiating low grade ESS from other mimickers such as endometrial stromal nodules, leiomyomas or cellular endometrial polyps, especially in curettage specimens, can be problematic.2,3,7 Many studies have shown that low grade ESS can exhibit various forms of histomorphologic changes, making their accurate diagnosis challenging. The identification of a smooth muscle component by smooth muscle markers, such as desmin and smooth muscle actin is not uncommon in ESS.23,24 The smooth muscle differentiated areas appear as white, firm foci on gross examination and form fascicles of slightly epithelioid cells microscopically. Other findings such as epithelioid appearance, sex-cord-like elements, endometrioid glandular elements and myxoid or fibroblastic differentiation have also been reported in low grade ESS.2,24,25 On the other end, differentiating undifferentiated ESSs from higher grade tumors including LMS, undifferentiated carcinoma, carcinosarcomas (or even less common tumors such as rhabdomyosarcoma, small cell carcinoma, lymphoma or primitive neuroectodermal tumors) could potentially be as problematic.1-6 Recent studies have noted that the soft tissue sarcomas showing smooth muscle differentiation tend to be more aggressive than those without the smooth muscle differentiation and thus the accurate differentiation is of great clinical significance.26,27 Accordingly dependable tests are needed that could differentiate between LMS and ESS with accuracy. The use of immunohistochemistry with a battery of markers including SMA, desmin, actin, h-caldesmon and CD10 has been proposed to be of value.10-15 However, the current IHC panel has been shown to be not entirely specific.12-14,16,17 Smooth muscle actin and desmin expression is noted in many different types of smooth muscle tumors in addition to myofibroblastic, skeletal muscle and myoepithelial tumors and thus lacks specificity.28,29 Similarly, h-caldesmon is quite specific for smooth muscle differentiation but lacks sensitivity.30,31 Other markers like Calponin, myogenin and myoD1 can sometimes be useful to assess smooth muscle differentiation but they too lack specificity as they are more sensitive and specific for myoepithelial, myofibroblast or skeletal muscle differentiation and less sensitive and specific for smooth muscle differentiation. CD10, postulated to be ESS-specific has been shown to be absent in up to one fourth of ESSs.12,13,15,17 Our results are in agreement with these findings. Cases with absent or decreased CD10 expression belonged to the undifferentiated ESS group.
Identifying genes expressed that differentiate between LMS and EMS is not only important in improving diagnostic accuracy, but could be relevant with respect to tumor biology. Using gene signature profiling, Davidson et al. have recently identified multiple genes that were differentially expressed in one tumor and not the other.22 Genes expressed in LMS included CDKN2A, FABP3, transgelin, JPH2, GEM, NAV2 and RAB23. While SLC7A10, EFNB3, CCND2, ECEL1, ITM2A, NPW, PLAG1 and GCGR genes were over expressed in ESS.22 Recent studies have shown that the t(7;17)(p15;q21) translocation is very common in typical ESSs.32 This translocation resulting in JAZF1-SUZ12 gene fusion was not observed in LMSs and in the majority of the undifferentiated ESSs.32 Although the precise function of transgelin remains unknown, recent studies have shown it to be involved in many biologic activities including regulating muscle fibers contractility, cell differentiation, tissue invasion and lately as a tumor suppressor.19,33-36 A recent study by Dos Santos Hildalgo et al. proposed a potential role of transgelin in the pathogenesis of endometriosis.37 They showed increased expression of the transgelin gene in endometriotic lesions compared with the eutopic endometrium of the same patients. Using real time polymerase chain reaction, they found no transgelin expression in eutopic proliferative and secretory endometrium of control and endometriotic patients.37 These studies lend support to our immunohistochemical findings of the lack of transgelin expression in normal and neoplastic endometrial stromal tissue. In the Davidson et al. study, the transgelin gene was not expressed in any of the 7 ESSs studied as compared to its overexpression in 13 uterine LMSs.22 The authors did not report the histologic grade or the metastatic status of any of the ESSs studied. Although they showed a picture with no transgelin expression by IHC, there was no mention of how many cases were evaluated by this method.22
In conclusion, transgelin appears to be a specific marker of smooth muscle differentiation in the uterus with 100% sensitivity and specificity and may be useful for distinguishing smooth muscle tumors from ESSs. It could be used as an additional marker useful for decision making, especially in those tumors with questionable immunophenotype and histology. Further studies are recommended to confirm our findings on a larger number of cases.
Acknowledgments
This work was supported in part by the NIH under Grant NIHR01HD0690431-01A1
REFERENCES
- 1.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CM, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . International Histological Classification of Tumours. IARC press; Lyon: 2003. [Google Scholar]
- 4.Evans HL. Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer. 1982;50:2170–2182. doi: 10.1002/1097-0142(19821115)50:10<2170::aid-cncr2820501033>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Amant F, Vergote I, Moerman P. The classification of a uterine sarcoma as “high-grade endometrial stromal sarcoma” should be abandoned. Gynecol Oncol. 2004;95:412–413. doi: 10.1016/j.ygyno.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine stromal neoplasms. A clinicopathological study of 117 cases. Am J Surg Pathol. 1990;14:415–438. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dionigi A, Oliva E, Clement PB, Young RH. Endometrial stromal nodules and endometrial stromal tumors with limited infiltration: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2002;26:567–581. doi: 10.1097/00000478-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kurihara S, Oda Y, Ohishi Y, et al. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol. 2008;32:1228–1238. doi: 10.1097/PAS.0b013e31816a3b42. [DOI] [PubMed] [Google Scholar]
- 9.Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–653. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 10.Kurihara S, Oda Y, Ohishi Y, et al. Coincident expression of beta-catenin and cyclin D1 in endometrial stromal tumors and related high-grade sarcomas. Mod Pathol. 2010;23:225–234. doi: 10.1038/modpathol.2009.162. [DOI] [PubMed] [Google Scholar]
- 11.de Leval L, Waltregny D, Boniver J, Young RH, Castronovo V, Oliva E. Use of histone deacetylase 8 (HDAC8), a new marker of smooth muscle differentiation, in the classification of mesenchymal tumors of the uterus. Am J Surg Pathol. 2006;30:319–327. doi: 10.1097/01.pas.0000188029.63706.31. [DOI] [PubMed] [Google Scholar]
- 12.Oliva E, Young RH, Amin MB, Clement PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002;26:403–412. doi: 10.1097/00000478-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Franquemont DW, Frierson HF, Jr, Mills SE. An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am J Surg pathol. 1991;15:861–870. doi: 10.1097/00000478-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lillemoe TJ, Perrone T, Norris HJ, Dehner LP. Myogenous phenotype of epithelial-like areas in endometrial stromal sarcomas. Arch Pathol Lab Med. 1991;115:215–219. [PubMed] [Google Scholar]
- 15.Chu PG, Arber DA, Weiss LM, Chang KL. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001;14:465–471. doi: 10.1038/modpathol.3880335. [DOI] [PubMed] [Google Scholar]
- 16.Aubry MC, Myers JL, Colby TV, Leslie KO, Tazelaar HD. Endometrial stromal sarcoma metastatic to the lung: a detailed analysis of 16 patients. Am J Surg Pathol. 2002;26:440–449. doi: 10.1097/00000478-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Abeler VM, Nenodovic M. Diagnostic immunohistochemistry in uterine sarcomas: a study of 397 cases. Int J Gynecol Pathol. 2011;30:236–243. doi: 10.1097/PGP.0b013e318200caff. [DOI] [PubMed] [Google Scholar]
- 18.Lees-Miller JP, Heely DH, Smillie LB, Kay CH. Isolation and characterization of an abundant and novel 22kDa protein (SM22) from chicken gizzard smooth muscle. J Biol Chem. 1987;262:2988–2993. [PubMed] [Google Scholar]
- 19.Assinder SJ, Stanton JA, Prasad PD. Transgelin: an actin-binding protein and tumor suppressor. Int J Biochem Cell Biol. 2009;41:482–486. doi: 10.1016/j.biocel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Robin YM, Penel N, Perot G, et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod Pathol. 2013;26:502–510. doi: 10.1038/modpathol.2012.192. [DOI] [PubMed] [Google Scholar]
- 21.Perot G, Derre J, Coindre JM, et al. Strong smooth muscle differentiation is dependent on myocardin gene amplification in most human retroperitoneal leiomyosarcomas. Cancer Res. 2009;69:2269–2278. doi: 10.1158/0008-5472.CAN-08-1443. [DOI] [PubMed] [Google Scholar]
- 22.Davidson B, Abeler VM, Hellesylt E, et al. Gene expression signatures differentiate uterine endometrial stromal sarcoma from leiomyosarcoma. Gynecol Oncol. 2013;128:349–355. doi: 10.1016/j.ygyno.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliva E, Clement PB, Young RH, Scully RE. Mixed endometrial stromal and smooth muscle tumors of the uterus: a clinicopathologic study of 15 cases. Am J Surg Pathol. 1998;22:997–1005. doi: 10.1097/00000478-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz A, Rush DS, Soslow RA. Endometrial stromal sarcomas with unusual histologic features: a report of 24 primary and metastatic tumors emphasizing fibroblastic and smooth muscle differentiation. Am J Surg Pathol. 2002;26:1142–1150. doi: 10.1097/00000478-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Tavasolli FA, Norris HJ. Mesenchymal tumors of the uterus. VII. A clinicopathological study of 60 endometrial stromal nodules. Histopathology. 1981;5:1–10. doi: 10.1111/j.1365-2559.1981.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher CD, Gustafson P, Rydholm A, Willén H, Akerman M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol. 2001;19:3045–3050. doi: 10.1200/JCO.2001.19.12.3045. [DOI] [PubMed] [Google Scholar]
- 27.Deyrup AT, Haydon RC, Huo D, et al. Myoid differentiation and prognosis in adult pleomorphic sarcomas of the extremity. Cancer. 2003;98:805–813. doi: 10.1002/cncr.11617. [DOI] [PubMed] [Google Scholar]
- 28.Folpe AL, Gown AM. Immunohistochemistry for analysis of soft tissue tumors. In: Weiss SW, Glodblum JR, editors. Enzinger and Weiss’s Soft Tissue Tumors. 5th edn. Mosby Elsevier; Maryland heights, MO: 2008. pp. 129–174. [Google Scholar]
- 29.Heim-Hall J, Yohe SL. Immunohistochemistry of soft tissue neoplasms. Arch Pathol Lab Med. 2008;132:476–789. doi: 10.5858/2008-132-476-AOITST. [DOI] [PubMed] [Google Scholar]
- 30.Miettinen MM, Sarlomo-Rikala M, Kovatich AJ, lasota J. Calponin and h-caldesmon in soft tissue tumors: consistent h-caldesmon immunoreactivity in gastrointestinal stromal tumors indicates traits of smooth muscle differentiation. Mod Pathol. 1999;12:756–762. [PubMed] [Google Scholar]
- 31.Hisaoka M, Wei-Qui S, Jian W, Morio T, Hashimoto H. Specific but variable expression of h-caldesmon in leiomyosarcomas: an immunohistochemical reassessment of a novel myogenic marker. Appl Imm Mol Morph. 2001;9:302–308. doi: 10.1097/00129039-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Chiang S, Oliva E. Recent development in uterine mesenchymal neoplasms. Histopathology. 2013;62:124–137. doi: 10.1111/his.12048. [DOI] [PubMed] [Google Scholar]
- 33.Lawson D, Harris M, Shapland C. Fibroblast transgelin and smooth muscle (SM22) are the same protein, the expression of which is downregulated in many cell lines. Cell Motil Cytoskel. 1997;38:250–257. doi: 10.1002/(SICI)1097-0169(1997)38:3<250::AID-CM3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Prasad PD, Stanton JA, Assinder SJ. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 2010;339:337–347. doi: 10.1007/s00441-009-0902-y. [DOI] [PubMed] [Google Scholar]
- 35.Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumours and RIE cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem. 2002;227:9790–9799. doi: 10.1074/jbc.M110086200. [DOI] [PubMed] [Google Scholar]
- 36.Abiges-Rizo C, Destaing O, Fourcade B, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.dos Santos Hidalgo G, Meola J, Rosa E Silva JC, Paro de Paz CC, Ferriani RA. TAGLN expression is deregulated in endometriosis and may be involved in cell invasion, migration, and differentiation. Fetil Steril. 2011;96:700–703. doi: 10.1016/j.fertnstert.2011.06.052. [DOI] [PubMed] [Google Scholar]