Fig 1. Localisation of PhoA proteins in M. gallisepticum cells.
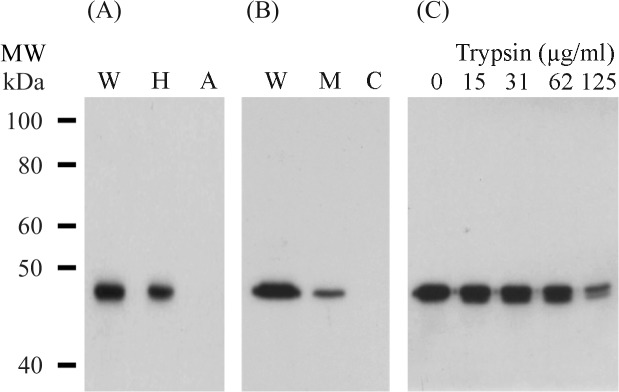
(A) Proteins of the pTGP3 transformant were separated into hydrophobic and aqueous fractions by Triton X-114 partitioning. Equivalent amounts of the fractions were separated by SDS-PAGE in 10% polyacrylamide gels, Western transferred and probed with a monoclonal antibody against alkaline phosphatase. Lanes: W, whole cells; H, hydrophobic fraction; A, aqueous fraction. The molecular weight (MW) markers were the biotinylated protein ladder from Cell Signaling Technology. (B) The membrane and cytosolic fractions of transformant pTGP3 were separated by SDS-PAGE in a 10% polyacrylamide gel and Western transferred. Immunostaining with a monoclonal antibody to alkaline phosphatase demonstrated the presence of PhoA in the whole cells (lane W) and the membrane fraction (lane M), but not in the cytosolic fraction (lane C). (C) Whole cells of the pTGP3 transformant were treated with increasing concentrations of trypsin. The cells in lane 0 were treated with TS buffer only. Proteins were separated by SDS-PAGE in 10% polyacrylamide gels and immunostained with a monoclonal antibody against alkaline phosphatase.
