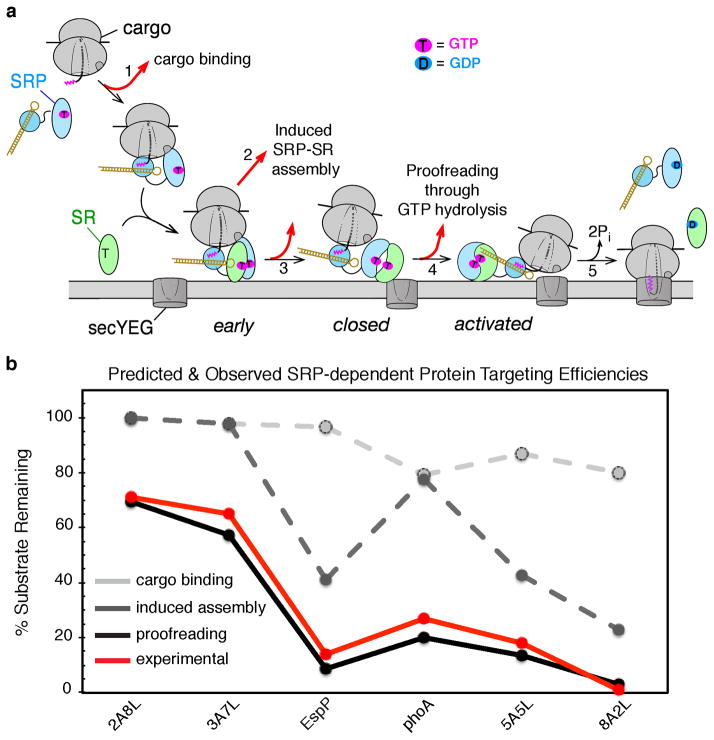
Figure 6.
A sequential series of checkpoints reject incorrect cargos from the SRP pathway. (a) Model for how conformational rearrangements in the SRP/SR GTPases provide the driving force and ensure the fidelity of protein targeting. Step 1, a cargo with a signal sequence (magenta) enters the pathway upon binding SRP. Step 2, the cargo-bound SRP forms a stabilized early intermediate with FtsY. Step 3, association of FtsY with membrane drives the rearrangements from the early intermediate to the closed complex. Step 4, the SecYEG translocon promotes conformational rearrangements that drive GTPase activation and cargo handover. Step 5, the cargo is unloaded from the SRP onto SecYEG, and GTP hydrolysis drives the disassembly and recycling of SRP and FtsY. At each step, the cargo can be either retained in (black arrows) or rejected (red arrows) from the pathway. Color codings are the same as in Figure 3. (b) Predicted fraction of cargos retained in the SRP pathway after cargo binding (light grey), induced SRP-SR assembly (dark grey), and kinetic proofreading through GTP hydrolysis (black). The experimentally determined protein targeting efficiencies are shown in red.