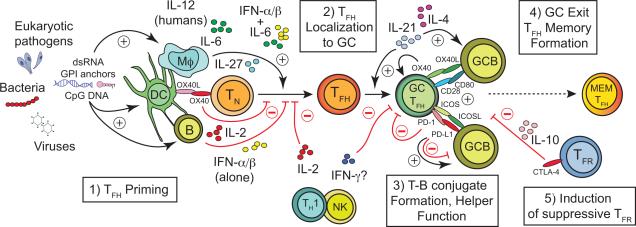
Figure 1.
Acute and chronic infections can impact five key processes that regulate the formation, function and persistence of pathogen-specific T follicular helper (TFH) cells. 1) Infections may induce or limit the activity or survival of unique subsets of antigen presenting cells (APC) bearing capacity to prime TFH responses. TFH priming may also be impacted by specific pathogen-associated molecular patterns (e.g. dsRNA, CpG DNA, glycophosphatidylinositol (GPI) anchors) that may elicit distinct cytokine profiles. APC-secreted cytokines can either promote (IL-12 in humans; IL-27, IL-6 or IL-6 + IFNα/β in mice) or constrain (IL-2 or IFNα/βalone) the differentiation of TFH cells from naïve CD4+ T cell precursors (TN). 2) Many infections cause dysregulation of chemokine expression that coordinates lymphocyte trafficking to or away from follicles (e.g. CXCL13 and CXCL12, CCL19, CCL21, respectively) or controls the architecture of lymphoid tissue (e.g. LTα/β, TNF), thereby limiting the magnitude or quality of TFH-orchestrated germinal center (GC) reactions. The capacity for TFH cells to traffic between GC reactions within a lymph node may also be impacted, although the relative contribution of inter-GC trafficking by TFH cells is less clear. 3) The capacity of TFH to form stable conjugates with antigen-presenting GC B cells (GCB) is necessary to sustain the GC reaction. Infections may alter the secretion of critical cytokines (IL-21, IL-4, IL-2, IFN-γ) or expression of cell surface receptors (ICOS, PD-1, CD80, OX40) and ligands (ICOSL, PD-L1, CD28, OX40L) that mediate these interactions and coordinate the bi-directional communication between TFH and GC B cells. 4) Infections likely impact the formation and stability of memory (MEM) TFH subsets, although the factors that regulate this are not yet clear. 5) Infections also likely trigger the expansion or enhance the suppressive capacity of Foxp3+ follicular regulatory (TFR) cells that impede the development of long-lived memory B cell and secreted antibody responses through specific cytokines (e.g. IL-10) or cell surface receptors and ligands (e.g. CTLA-4). Black arrows and red “T” lines represent factors that either promote or limit TFH and GC B cell responses, respectively.