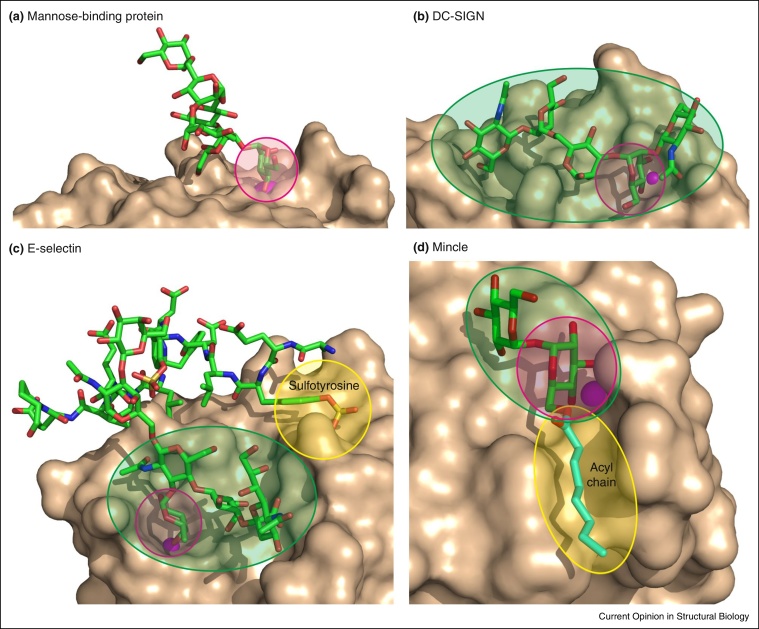
Figure 2.
Multiple different ways in which binding specificity of C-type carbohydrate-recognition domains is enhanced by extended and accessory binding sites. Each of the binding sites involves a primary interaction between the Ca2+, shown in magenta, and two adjacent hydroxyl groups on a monosaccharide residue. (a) The relatively open binding site in mannose-binding protein binds only a terminal mannose residue, so only this residue interacts with the protein (2MSB). (b) DC-SIGN binds a more complex Man3GlcNAc2 oligosaccharide through an extended binding site that accommodates sugars on either side of the mannose residue in the primary binding site (1K9J). (c) In addition to ligation of fucose to Ca2+, the sialyl Lewisx oligosaccharide interacts with an extended binding site in E-selectin, which also has an accessory binding site for sulfated tyrosine residues on a glycoprotein ligand (1G1S). (d) Mincle binds to the disaccharide trehalose as a result of one glucose residue binding in the Ca2+ site and the second glucose residue contacting an adjacent site. In addition, glycolipid binding is enhanced through an accessory site that forms a hydrophobic grove which can interact with acyl chains on the 6-OH groups of the glucose residues (4KZV). Primary binding sites are highlighted in pink, extended oligosaccharide-binding sites are indicated in green and accessory sites for other modifications are shaded yellow.