Abstract
Fish oil recovered from fresh water fish visceral waste (FVW-FO) through lactic acid fermentation (FO-LAF) and enzymatic hydrolysis (FO-EH) were fed to rats to study their influence on lipid peroxidation and activities of antioxidant and membrane bound enzyme in liver, heart and brain. Feeding of FO-LAF and FO-EH resulted in increase (P < 0.05) in lipid peroxides level in serum, liver, brain and heart tissues compared to ground nut oil (control). Activity of catalase (40–235 %) and superoxide dismutase (17–143 %) also increased (P < 0.05) with incremental level of EPA + DHA in diet. The increase was similar to cod liver oil fed rats at same concentration of EPA + DHA. FO-LAF and FO-EH increased (P < 0.05) the Na+K+ ATPase activity in liver and brain microsomes, Ca+Mg+ ATPase in heart microsome and acetylcholine esterase in brain microsomes when fed with 5 % EPA + DHA. There was also significant change in fatty acid composition and cholesterol/phospholipid ratio in microsomes of rat fed with FVW-FO. Feeding FVW-FO recovered by biotechnological approaches enhanced the activity of antioxidant enzymes in tissues, modulates the activities of membrane bound enzymes and improved the fatty acid composition in microsomes of tissues similar to CLO. Utilization of these processing wastes for the production of valuable biofunctional products can reduce the mounting economic values of fish oil and minimize the environmental pollution problems.
Keywords: FVW, Fermentation, Lipids, Antioxidant enzymes, Na+K+ ATPase, Fatty acid
Introduction
Currently the world fish production has been estimated to be around 141 million metric tonnes (Amit et al. 2011). Considering 45 % of the live weight is waste, it is estimated that nearly 63.6 million metric tonnes of waste is generated globally. As the constraints related to environmental issues enforced by legislation are becoming quite stringent, it is necessary to utilize these byproducts generated by fish processing industries. Owing to this and mounting economic value of fish oil (Turchini et al. 2009) intensive research is being conducted globally for the utilization of fish waste (Amit et al. 2011; Rustad 2003). Lipid content in fish processing waste (FPW), including fresh water fish processing, have been reported to be in the range of 4–43.8 % (Turchini et al. 2009; Amit et al. 2012). Fish oil recovered from FPW can fulfill the requirement of fish oil by the aqua feed industry and which may also serve as sustainable alternatives to fish oil for human consumption.
Fish oil is of prime nutritional/pharmaceutical importance due to its n-3 fatty acids, particularly EPA and DHA. Consumption of PUFA has been shown to decrease plasma lipids and exhibit anti-inflamatory effects on endothelium resulting in improvement of vascular function (Caterina et al. 2000; Christon 2003). PUFA are also susceptible to lipid peroxidation and the product of which may have deleterious effects leading to tissue damage (Shireen et al. 2008; Reena and Lokesh 2011). The susceptibility of cell to oxidative damage is affected by the efficiency of antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase and glutathione transferase (Ramaprasad et al. 2005). If the antioxidant defense system fails to protect the cell from damage, it may affect the tissue and membrane functions (Sandhir et al. 1994).
The compositions of lipids in membrane are known to affect a wide variety of membrane properties such as activities of membrane bound enzymes, receptors, carriers or ion channels. Membrane fatty acid composition can be modulated through dietary feeding of essential fatty acids that coordinates the structure and function of the membrane system (Horrocks and Farooqui 2004). Kumosani and Moselhy (2011) showed that dietary cod liver oil increases the activity of membrane bound enzyme in rat’s brain. Apart from beneficial effect of n-3 fatty acids the higher degree of unsaturation can also increase the lipid peroxidation, which can directly inhibit the activities of membrane bound enzymes such as Na+K+ ATPase (Sandhir et al. 1994).
Earlier, we have shown the effectiveness of LAF (Amit et al. 2011) and EH (Swapna et al. 2011) for the recovery of lipids rich in n-3 fatty acids from fresh water FVW. Lipids recovered by these biotechnological approaches have also shown to possess anticholesterol properties (Amit et al. 2013). This study hypothesizes that FO recovered from FVW may affect the susceptibility of tissues to oxidative stress that may be compensated by activating the antioxidant defense system in rodent model. Since n-3 fatty acids affect the activity of membrane bound enzymes (Kumosani and Moselhy 2011; Gerbi et al. 1999) feeding diet containing FVW-FO is expected to modulate the activities of membrane bound enzymes. This study provides a new insight into the possible application of FVW-FO for health benefits similar to CLO (cod liver oil). Further, development of solvent free biotechnological approaches like LAF and EH for utilization of fresh water FVW for recovery of lipids and studies on its bioactivity can bring additional value from the waste. Thus, the aim of the study was to evaluate the effect of FVW-FO on lipid peroxidation, antioxidant enzymes and membrane bound enzymes in rats.
Materials and methods
Fresh water fish visceral waste (FVW) obtained by dressing of freshly harvested Indian major carps (Rohu – Labeo rohita; and Catla – Catla catla) were procured from local market and transported to laboratory in sterile pouches under iced conditions and kept as such until further use. Lactic acid bacteria (LAB) culture was maintained on Mann–Rogosa–Sharpe (MRS) agar (Hi-Media, Mumbai, India) slants stored at 4 °C and sub-cultured periodically. D-glucose and sodium chloride for the fermentation experiments were purchased from M/s Loba chemicals (Mumbai, India). Food grade casein, cellulose, sucrose, methionine, vitamins, minerals and choline chloride were purchased from Hi-media Laboratories Pvt Ltd. (Mumbai, India). Standard rat feed was purchased from M/S. Sai Durga Feeds and Foods (Bangalore, India). All other chemicals and solvents used were of analytic grade, unless otherwise mentioned.
Fermentation of FVW
The LAB culture P. acidilactici NCIM5368 was grown in 100 ml of MRS-Broth for 24 h at 37 ± 1 °C in a shaking incubator (Technico Ltd., India) at 100 rpm. The cells were harvested by centrifuging (C31 Cooling centrifuge, Remi-India, Mumbai, India) at 5000 × g for 10 min, the cell pellets were washed twice and resuspended in sterile physiological saline (100 ml). The viable cells, after serial dilution, were assayed by counting colony forming units (CFU) on MRS agar plates and it is in the range of 8.1 to 8.4 log CFU/ml.
Fermentation of FVW was carried out according to Amit et al. (2011). In brief, FVW of catla and rohu were mixed in the ratio of 1:1 (w/w) and minced in a Waring blender (Stephan Mill, UM5 Universal, Hong Kong) and cooked at 85 ºC for 15 min to inactivate the endogenous enzymes and native microflora. The sample was mixed with 10 % dextrose and 2 % NaCl (both w/w) by continuous stirring followed by the addition of 24 h old LAB inoculum at 10 % (v/w). The mix (∼5 kg) was placed in a sterile airtight container (10 liter bottle) and incubated for 48 h at 37 ± 1 °C. On post incubation, the slurry was centrifuged at 6000 × g for 20 min to separate the fermented mass into three phases viz., an upper layer of oil, middle layer of fermented liquor with hydrolyzed proteins and a bottom residue rich in collagen. The upper fish oil layer was used for feeding experiments.
Enzymatic hydrolysis of FVW
EH of FVW was carried out according to Swapna et al. (2011). In brief, the FVW was minced in a Waring blender followed by cooking at 85 °C for 15 min to inactivate the endogenous enzymes. Hydrolysis of cooked FVW was carried out using Protease-P-amano® (P Amano6; 60,000 U/g). Briefly, the cooked FVW (5 kg) was mixed with water (1:1 w/v), homogenized after addition of enzymes (0.5 %, w/w of FVW) and incubated for 2 h at 40 °C with shaking after every 10 min. The resulting sample was centrifuged at 6000×g for 20 min, the upper oil layer was collected and used for the feeding experiment.
Animal diets
The animal feeding trials had the prior approval of the animal ethic committee of the institute. The use and care of rats followed all the necessary protocols to ensure the humane treatment of the animals. Male Albino rats (OUTB- wistar, IND-CFT (2c), (n = 48) with mean ± SD weight of 40 ± 2 g were housed in steel cages at RT (28 ± 2 °C) with a 12 h dark /light cycle. Rats were fed with fresh-pelleted diet and had free access to water for 7 days of acclimatization. After 7 days, rats were randomly assigned to 8 groups (n = 6/group) and groups 1–6 were fed with FVW-FO at different concentration of EPA + DHA (1.25 %, 2.5 %, 5 % EPA + DHA) recovered through LAF and EH for 8 weeks. Rats received diet-containing GNO as the sole source of fat served as control while the group fed with CLO (2.5 % EPA + DHA) was the positive control. Rats were fed fresh diet daily and leftover diets were weighed and discarded. The rats had free access to food and water throughout the study. Daily feed intake and weekly gain in body weight was recorded. The ingredients used in the basal diet were (g/100 g): casein 20; cellulose 5; sucrose 60; AIN-76 mineral mix 3.5; AIN-76 vitamin mix 1; methionine 0.3; choline chloride 0.2; and fat 10. On completion of the experiment, rats were anesthetized with diethyl ether and sacrificed by exsanguination. Blood was collected by cardiac puncture and serum was seperated by centrifugation at 1100×g for 30 min. Liver, heart and brain were removed and rinced with ice-cold saline, blotted, weighed and stored at – 20 ºC until analysis.
Lipid extraction and analysis of fatty acids
Total lipid from tissues was extracted according to the method of Folch et al. (1957). Phospholipids were measured by the method of Stewart (1980) using dipalmitoylphosphatidylcholine as reference standard. Fatty acid composition was analyzed as methyl esters (Morrison and Smith 1963) by gas chromatography (Shimadzu GC 2014; M/s Shimadzu, Kyoto, Japan) fitted with a flame ionization detector for identifying the individual fatty acids. Fatty acid methyl ester dissolved in hexane was analyzed on a fused silica capillary column (30 m × 0.32 mm × 0.25 μm; Omegawax™ 320; M/s Supelco, Bellefonte, PA, USA) with a split ratio of 1:30. The temperatures of injector, column, and detector were set at 250 °C, 200 °C, and 260 °C, respectively. The fatty acids were identified by comparing with authentic standards.
Preparation of tissue samples
Liver, brain and heart (1 g) homogenates were prepared at 4 ºC in 30 mM phosphate buffer (pH 7.2) for lipid peroxides and enzyme assay. The homogenate was centrifuged at 10000×g for 10 min at 4 ºC to remove nuclei and cell debris. The supernatant was used for antioxidant enzyme assays. Microsomes from different organs were prepared according to the procedure of Yehuda and Shamir (1971) from a 10 % homogenate in 0.25 M sucrose, 0.03 M histidine and 0.001 M EDTA (pH 7.4).
Lipid peroxidation
Lipid peroxides in serum and tissue homogenate was estimated using TBA (Okhawa et al. 1979). Briefly, 20 % acetic acid (1.5 ml), 8 % sodium dodecyl sulphate (0.2 ml) and 0.8 % TBA (1.5 ml) were added to the sample in that order, incubated for 1 h in constantly boiling water (97 ± 2 ºC), allowed to cool followed by the addition of n-butanol (5 ml) and centrifuged at 3000×g for 15 min. The upper butanol phase containing TBA reactive substances (TBARS) was read spectrofluorometrically (Hitachi, Japan) with an excitation at 515 nm and emission at 553 nm. 1,1,3,3-Tetramethoxypropane (TMP) was used as a standard to estimate the TBARS.
Antioxidant enzymes
Activity of CAT in tissues was determined by measuring the decrease in absorption at 240 nm in a reaction mixture containing phosphate buffer (PB) (0.1 mM, pH 7.0) and H2O2 (8.8 mM) according to the procedure described by Aebi (1984). One CAT unit is defined as the amount of enzyme required to decompose 1 M of H2O2 /min. SOD activity was measured by the inhibition of cytochrome C reduction mediated via superoxide anions generated by xanthine-xanthine oxidase and monitored at 550 nm (Flobe and Oting 1984). One unit of SOD is defined as the amount-required to inhibit the reduction of cytochrome C by 50 %. GST activity in tissue homogenates was determined following the formation of conjugate of reduced glutathione (GSH) and 1-chloro-dinitrobenzene at 340 nm in a reaction mixture containing GSH (20 mM), CDNB (20 mM), PB (0.1 mM, pH 6.5) (Gluthenberg et al. 1985).
Na+K+-ATPase and Ca+Mg+ ATPase in liver, heart and brain microsomes
Na+K+-ATPase and Ca+Mg+ ATPase activity were estimated by the method of Kaplay (1978). Briefly, buffer composition used for the assay was as follows: MgCl (3 mM), KCl (14 mM), NaCl (140 mM)/ CaCl2 (140 mM) for Ca+Mg+ ATPase, EDTA (0.2 mM), and Tris– HCl (20 mM, pH 7.4). For Na+K+-ATPase, samples were simultaneously run in two batches, one containing inhibitor ouabain (2 mM) and the other without. The sample blank containing no assay standard and experimental sample (microsomes) was also run simultaneously. After incubation for 60 min, the reaction was stopped by the addition of trichloroacetic acid (10 %). Inorganic phosphate (Pi2) liberated was determined in aliquots (0.7 ml) of incubated mixtures by the addition of ascorbic acid-ammonium molybdate solution (0.3 ml) (Ames 1966). Then, the reaction mixture was mixed well and incubated at 45 ºC for 20 min. Extinction at 820 nm was measured by UV-visible spectrophotometer (Shimadzu 1601, Kyoto, Japan). Specific activity was expressed as mol Pi /h/mg protein. Similarly, total Ca+Mg+ ATPase was determined by measuring the inorganic phosphate released from the hydrolysis of ATP in the presence of CaCl2.
Acetylcholine esterase activity in brain microsomes
Acetylcholine esterase (AchE) activity in brain microsomes was determined according to the method described by Ellman et al. (1961). A cocktail containing 13 ml of 1 M NaCl, 2 ml of 1 M MgCl2, 10 ml of 0.5 M Tris–HCl (pH 7.5) and 10 ml of 0.2 M EDTA was prepared. Other reagents included 1 mM DTNB and the substrate 0.1 M acetylthiocholine chloride. The reaction mixture contained 10.5 ml of the cocktail, 3 ml DTNB and 6.5 ml double distilled water. To the reaction mixture (2 ml), 30 μl of 0.1 M acetylthiocholine chloride and brain microsomes containing the enzyme (10 μg) were added and the change in optical density was measured at 412 nm over a period of 5 min. Specific activity was calculated using the molar coefficient for –SH group of DTNB.
Statistical analysis
The data obtained in the experiments were subjected to analysis of variance (ANOVA) and whenever significant, mean separation was done by Duncan’s multiple range test at a confidence level of 95 % (i.e., p < 0.05). All the statistical analysis were done by using STATISTICA software (Statsoft 1999).
Results
Fatty acid composition of control and experimental diet is given in Table 1. FO-LAF and FO-EH were added to diet to provide EPA + DHA content of 1.25, 2.5, 5 %. No n-3 fatty acids were detected in the control diet. The n-6/n-3 ratio of diet containing FO-LAF and FO-EH at 1.25, 2.5 and 5.0 % EPA + DHA levels were 4.01, 2.08, 1.37 and 4.18, 1.94, 1.35, respectively. There were no significant (p > 0.05) difference in food intake, gain in body weight, food efficiency ratio, organ weights (liver, brain, heart, kidney and spleen) and biochemical measurements (total protein, albumin, globulin and bilirubin) in serum, in rats fed with different concentration of either FO-LAF or FO-EH compared to control group (data not shown). Further, no significant (p > 0.05) changes were observed on hematological parameters such as white blood cells count, red blood cells count, hemoglobin content, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration and platelets count concluding no harmful effects to rats in feeding FO-LAF and FO-EH (data not shown).
Table 1.
Fatty acid composition (%) of control and experimental diet
| Fatty acids (%) | FO-LAF | FO-EH | GNO | CLO-2.5 | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25 | 2.50 | 5.0 | 1.25 | 2.50 | 5.0 | |||
| 14:0 | 1.50 | 2.34 | 3.31 | 1.40 | 2.74 | 3.72 | ND | 2.37 |
| 16:0 | 23.60 | 26.78 | 24.73 | 22.6 | 23.78 | 24.75 | 15.98 | 17.65 |
| 16:1n-7 | 9.30 | 10.22 | 11.31 | 10.40 | 10.22 | 10.90 | ND | 1.45 |
| 18:0 | 5.90 | 5.02 | 6.75 | 5.50 | 5.02 | 5.10 | 2.23 | 14.4 |
| 18:1n-9 | 21.33 | 19.53 | 17.63 | 22.84 | 21.53 | 16.30 | 28.30 | 30.03 |
| 18:1n-7 | 7.52 | 3.94 | 2.05 | 5.10 | 3.94 | 4.10 | 11.10 | ND |
| 18:2n-6 | 19.49 | 16.40 | 14.19 | 19.30 | 16.60 | 14.96 | 35.70 | 23.71 |
| 18:3n-3 | 3.90 | 6.37 | 8.14 | 3.60 | 6.87 | 8.89 | ND | ND |
| 20:0 | 0.56 | 1.17 | 0.90 | 0.60 | 1.42 | 0.85 | 1.49 | 1.05 |
| 20:1n-9 | 0.79 | 1.23 | 0.75 | 0.80 | 1.32 | 0.70 | ND | 1.24 |
| 20:4n-6 | 1.66 | 2.84 | 3.96 | 1.29 | 2.52 | 3.86 | ND | 1.60 |
| 20:5n-3 | 0.73 | 1.48 | 2.36 | 0.88 | 1.23 | 2.49 | ND | 1.46 |
| 22:6n-3 | 0.54 | 1.12 | 2.68 | 0.44 | 1.32 | 2.52 | ND | 1.05 |
| EPA + DHA | 1.27 | 2.60 | 5.14 | 1.32 | 2.55 | 5.01 | ND | 2.51 |
FO-LAF fish oil recovered by lactic acid fermentation; FO–EH fish oil recovered by enzymatic hydrolysis; GNO groundnut oil; CLO cod liver oil; 1.25, 2.50. 5.0 – percentage of EPA + DHA in diet; ND – not detected
Lipid peroxides and antioxidant enzymes in serum
Rats fed with FVW-FO resulted in significant increase in lipid peroxides and antioxidant enzymes in serum (Table 2). Lipid peroxides level in serum of FO-LAF and FO-EH fed groups increased by 36.4–209 % and 27.2–218 %, respectively, compared to control rats (Table 2). Compared to GNO group activity of antioxidant enzymes was significantly increased with an incremental level of EPA + DHA in diet (Table 2). CAT and SOD activity in serum of FO-FVW fed groups were dose dependently higher by 44–144 and 87–253 % respectively, compared to control group. However, there was no significant (p > 0.05) difference in lipid peroxides and activities antioxidant enzymes between FO-LAF, FO-EH and CLO fed groups at same concentration of EPA + DHA.
Table 2.
Lipid peroxidation and activity of antioxidant enzymes in serum of rats fed with n-3 fatty acids recovered from fish visceral waste through biotechnological approaches at incremental levels
| Groups | Lipid peroxidation (nmol/mg protein) | Catalase (μmol/min/mg protein) | Superoxide dismutase (unit/min/mg protein) |
|---|---|---|---|
| FO-LAF-1.25 | 0.15 ± 0.02a | 0.37 ± 0.03a | 2.45 ± 0.20a |
| FO-LAF-2.50 | 0.22 ± 0.03b | 0.47 ± 0.05b | 3.59 ± 0.10b |
| FO-LAF-5.0 | 0.34 ± 0.05c | 0.61 ± 0.03c | 4.63 ± 0.28 c |
| FO-EH-1.25 | 0.14 ± 0.01a | 0.36 ± 0.05a | 2.57 ± 0.32a |
| FO-EH -2.50 | 0.20 ± 0.03b | 0.51 ± 0.04b | 3.40 ± 0.46b |
| FO-EH -5.0 | 0.35 ± 0.04c | 0.60 ± 0.03c | 4.53 ± 0.33c |
| CLO-2.5 | 0.21 ± 0.02b | 0.53 ± 0.02b | 3.48 ± 0.21b |
| GNO | 0.11 ± 0.01d | 0.25 ± 0.02d | 1.31 ± 0.31d |
FO-LAF fish oil recovered by lactic acid fermentation; FO–EH fish oil recovered by enzymatic hydrolysis; GNO groundnut oil; CLO cod liver oil, 1.25, 2.50. 5.0 – percentage of EPA + DHA in diet; Data represent the mean ± SD, values not sharing common superscript within the column are significantly different (p < 0.05)
Lipid peroxides and antioxidant enzymes in liver, brain and heart
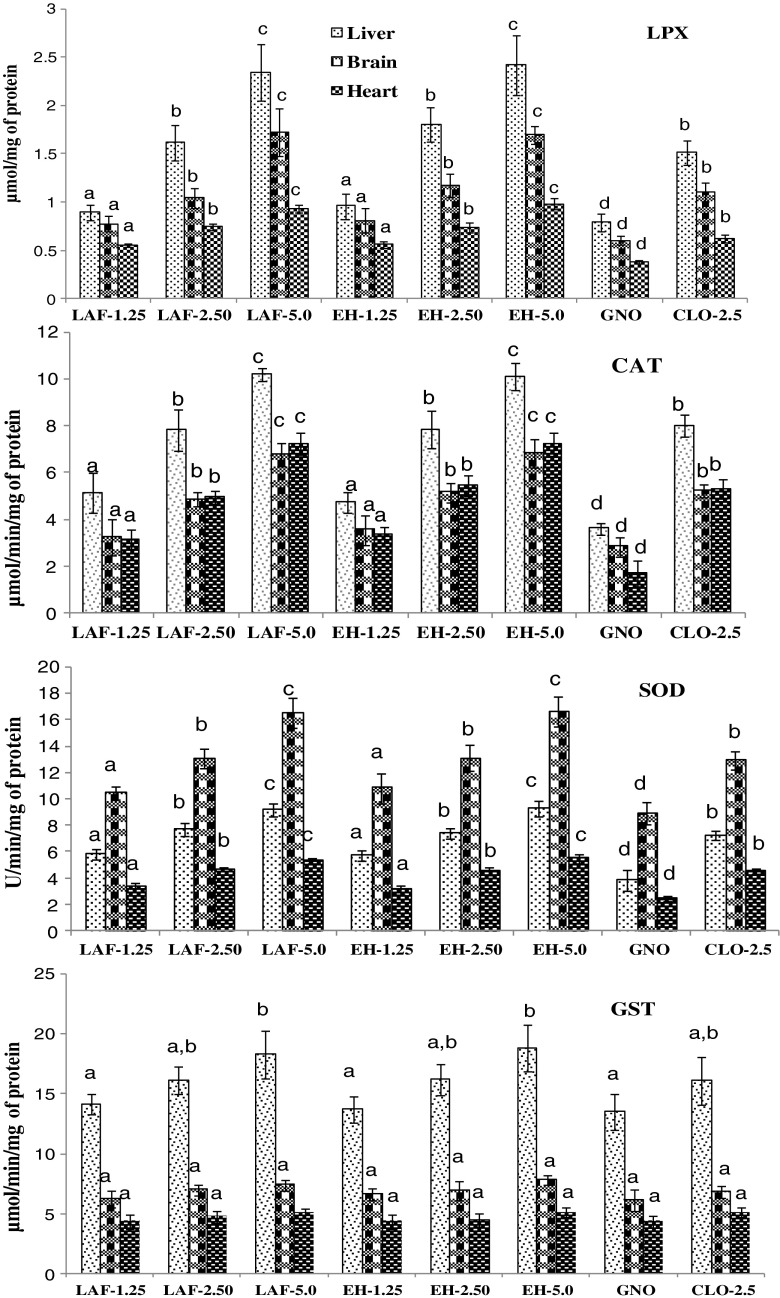
The rats fed with FO-LAF and FO-EH showed significant increase in lipid peroxides and activities of antioxidant enzymes (CAT, SOD and GST) compared to control group (GNO) in liver, brain and heart (Figure 1). At incremental level of EPA + DHA in the FO-LAF incorporated diet, the lipid peroxide level in liver, brain and heart increased by 31–200, 28–186, and 44–144 %, respectively, compared to control rats (Figure 1). Similarly, lipid peroxides level in liver, brain and heart of rats fed with FO-EH also showed increase by 40–209, 34–182, 45–156 % respectively, compared to control rats.
Fig. 1.
Lipid peroxidation (LPX), activity of catalase (CAT), superoxide dismutase (SOD) and Glutathione transferase (GST) in liver, heart and brain of rats fed with incremental levels of FVW-FO recovered by biotechnological approaches. FO-LAF – fish oil recovered by lactic acid fermentation; FO–EH – fish oil recovered by enzymatic hydrolysis; GNO – groundnut oil (control); CLO- cod liver oil; 1.25, 2.50, 5.0 – percentage of EPA + DHA in diet; values not sharing common alphabets within same pattern are significantly different (p < 0.05).
The activity of CAT and SOD were increased significantly (P < 0.05) in FO-LAF and FO-EH fed groups with 1.25, 2.50 and 5.0 % EPA + DHA in the diet (Figure 1). The increase in CAT activity in liver, brain and heart of rats fed diets containing 1.25, 2.50 and 5.0 %, EPA + DHA from FVW-FO were found to be 40–204, 41–199, and 46–235 %, respectively compared to control rats. Similarly, activity of SOD in liver, brain and heart of rats fed with FVW-FO at 1.25, 2.50 and 5.0 % of EPA + DHA was higher by 50–143, 17–87, 27–116 % respectively, compared to control rats. The activity of GST in liver was significantly higher in FVW-FO fed groups compared to control while no significant change was observed in heart and brain (Figure 1). Although FVW-FO obtained by fermentation and enzymatic hydrolysis affected the activities of antioxidant enzymes compared to control, there was no difference between these groups and CLO fed group when fed with same concentration of EPA and DHA.
Membrane bound enzyme activity in liver, brain and heart
Results on the effect of feeding rats with FO-LAF, FO-EH and CLO incorporated diets on Na+K+ ATPase and Ca+Mg+ ATPases in liver and heart microsomes and AChE and Na+K+ ATPase in brain microsomes are given in Table 3. Results reveal a significant (P < 0.05) increase in Na+K+ ATPase activity in the liver microsomes compared to control group (Table 3). However, no significant change was found in the activity of Na+K+ ATPase in the heart microsomes of rats fed with FO-LAF and FO-EH at 1.25, 2.50 and 5.0 % of EPA + DHA compared to control (Table 3). In rats fed with 5 % EPA + DHA there was significant (P < 0.05) increase in Na+K+ ATPase and Ca+Mg+ ATPases activity in brain and heart microsomes, respectively. Results indicated that effect of FVW-FO on membrane bound enzymes is tissue specific and the effect was similar between FO-LAF, FO-EH and CLO fed groups. The AChE activity in brain microsomes exhibited a marginal increase in its activity with incremental level of EPA + DHA in the diet, however the activity was significantly higher in groups fed with 5.0 % of EPA + DHA compared to control. No significant difference was found in activity of brain AChE between groups fed with FO-LAF, FO-EH and CLO.
Table 3.
Membrane bound enzymes in liver, heart and brain of rats fed with n-3 fatty acids recovered from fish visceral waste through biotechnological approaches at incremental levels
| Groups | Liver | Heart | Brain | |||
|---|---|---|---|---|---|---|
| Na+/K+ ATPase* | Ca+/Mg+ ATPase* | Na+/K+ ATPase* | Ca+/Mg+ ATPase* | Na+/K+ ATPase* | AChE# | |
| FO-LAF-1.25 | 7.48 ± 0.60a | 6.98 ± 0.38a | 4.41 ± 1.39a | 6.98 ± 0.40a,b | 67.68 ± 3.67a,c | 6.86 ± 1.87a,b |
| FO-LAF-2.50 | 8.09 ± 0.78a,b | 7.72 ± 0.71a | 5.09 ± 0.86a | 7.93 ± 0.82b,c | 70.35 ± 4.27a,c | 7.84 ± 0.56a,b |
| FO-LAF-5.0 | 8.85 ± 0.58b | 8.28 ± 0.42a | 6.29 ± 1.74a | 8.60 ± 0.59c | 72.45 ± 3.08c | 8.97 ± 0.62b |
| FO-EH-1.25 | 7.46 ± 0.63a | 7.15 ± 0.76a | 4.24 ± 1.39a | 7.02 ± 0.64a,b | 67.95 ± 3.53a,c | 6.74 ± 1.14a |
| FO-EH -2.50 | 8.19 ± 0.82a,b | 8.01 ± 0.87a | 5.52 ± 1.92a | 8.01 ± 0.47b,c | 69.90 ± 5.49a,c | 7.80 ± 0.65a,b |
| FO-EH -5.0 | 8.97 ± 0.31b | 8.41 ± 1.08a | 6.02 ± 1.11a | 8.56 ± 0.67c | 72.02 ± 2.21c | 8.84 ± 0.63b |
| CLO-2.5 | 8.49 ± 0.82b | 8.21 ± 0.78a | 5.72 ± 1.62a | 8.22 ± 0.37c | 70.90 ± 2.49c | 8.34 ± 0.48b |
| GNO | 6.72 ± 0.67a | 6.98 ± 0.92a | 4.07 ± 1.20a | 6.01 ± 0.70a | 64.42 ± 3.07a | 6.31 ± 1.06a |
AChE acetyl choline esterase; FO-LAF fish oil recovered by lactic acid fermentation; FO–EH fish oil recovered by enzymatic hydrolysis; GNO groundnut oil; CLO cod liver oil; 1.25, 2.50. 5.0 – percentage of EPA + DHA in diet; “*” – expressed as mol Pi/h/mg of protein; “#” - μmol/min/mg of protein; Data represent the mean ± SD, values not sharing common superscript within the column are significantly different (p < 0.05)
Fatty acid profile of microsome of liver, brain and heart
Fatty acid profile in liver, brain and heart microsomal lipids of rats fed diet incorporated with FO-LAF, FO-EH and CLO are given in Table 4. The EPA + DHA levels in liver microsome of rats fed with diets containing EPA + DHA at 1.25, 2.50 and 5.0 % from either of FO-LAF and FO-EH were found to be 1.87, 3.81, 6.86 % and 1.92, 4.38, 6.67 % respectively. EPA and DHA were not detected in control groups indicating their presence in liver microsomes of FO fed groups is due to the dietary EPA + DHA content. The initial level of DHA in brain microsomes of GNO fed group was 5.04 % and its level raised to 6.5, 7.7, 9.7 % and 6.4, 7.25, 9.3 % on feeding EPA + DHA at 1.25, 2.5 and 5.0 % from FO-LAF and FO-EH, respectively (Table 4). The increase in n-3 fatty acids (EPA + DHA) in brain was accompanied by marked decline in n-6 fatty acids. The fatty acid profile of the heart microsomes was also significantly altered on feeding varying levels of EPA + DHA (Table 4). EPA was not detected in the heart microsomes of control rats, but its level rose to 3.97, 6.86, 9.05 % and 3.95, 6.65, 8.41 % on feeding incremental levels of EPA + DHA in diet from FO-LAF and FO-EH respectively. The initial level of DHA in the heart tissue of control rats was 2.01 % of total fatty acids and was raised to 2.8, 4.51, 6.04 % and 2.70, 4.20, 5.80 %, after feeding dietary EPA + DHA at 1.25, 2.50 and 5.0 % from FO-LAF and FO-EH respectively. Results demonstrate that feeding fish oil at incremental levels of EPA + DHA significantly (p < 0.05) increased their levels in the liver, brain and heart microsomes, which was similar to cod liver oil.
Table 4.
Liver, brain and heart microsomes fatty acid profile (%) of rats fed with n-3 fatty acids recovered from fish visceral waste through biotechnological approaches at incremental levels
| Fatty acids | FO-LAF | FO-EH | GNO | CLO | ||||
|---|---|---|---|---|---|---|---|---|
| 1.25 | 2.50 | 5.0 | 1.25 | 2.50 | 5.0 | 2.5 | ||
| Liver microsomes | ||||||||
| SFA | 35.5 ± 2.6a | 34.1 ± 2.9a | 33.2 ± 3.0a | 34.8 ± 2.8a | 33.2 ± 3.4a | 32.3 ± 2.6a | 35.8 ± 3.8a | 37.4 ± 4.1a |
| MUFA | 32.7 ± 3.1a | 33.9 ± 4.1a | 35.6 ± 2.7a | 33.3 ± 2.2a | 35.4 ± 4.0a | 34.5 ± 3.7a | 33.7 ± 2.5a | 31.5 ± 3.7a |
| 18:2n-6 | 13.7 ± 1.1a | 10.84 ± 1.4b | 8.26 ± 0.9c | 13.6 ± 1.0a | 10.2 ± 1.5b | 6.2 ± 1.4c | 16.3 ± 1.1d | 13.71 ± 1.8a |
| 20:4n-6 | 9.7 ± 0.7a | 7.22 ± 0.8b | 5.04 ± 0.4c | 8.9 ± 0.6a | 7.5 ± 0.6b | 5.8 ± 0.4c | 12.0 ± 1.2d | 7.44 ± 0.9b |
| 20:5n-3 | 0.8 ± 0.1a | 1.63 ± 0.3b | 2.9 ± 0.4c | 0.9 ± 0.2a | 2.12 ± 0.4b | 3.22 ± 0.5c | ND | 1.79 ± 0.5b |
| 22:5n-3 | 0.56 ± 0.2a | 0.71 ± 0.1a | 0.75 ± 0.2a | 0.53 ± 0.1a | 0.58 ± 0.1a | 0.66 ± 0.1a | ND | 0.64 ± 0.1a |
| 22:6n-3 | 1.07 ± 0.3a | 2.18 ± 0.2b | 3.96 ± 0.2c | 1.02 ± 0.1a | 2.26 ± 0.3b | 3.65 ± 0.2c | ND | 2.88 ± 0.2d |
| Brain microsomes | ||||||||
| SFA | 41.27 ± 3.3a | 40.63 ± 3.6a | 39.54 ± 4.2a | 41.04 ± 2.9a | 39.6 ± 3.2a | 39.2 ± 1.8a | 41.3 ± 3.5a | 38.3 ± 3.2a |
| MUFA | 26.4 ± 1.4a | 25.4 ± 3.1a | 25.6 ± 2.4a | 24.4 ± 2.2a | 26.1 ± 1.7a | 27.4 ± 1.9a | 28.5 ± 1.6a | 30.5 ± 1.6a |
| 18:2n-6 | 16.4 ± 1.2a | 12.5 ± 1.1b | 9.1 ± 0.7c | 16.1 ± 1.3a | 11.2 ± 0.8b | 8.6 ± 0.6c | 18.8 ± 1.5a | 10.8 ± 1.6b,c |
| 20:4n-6 | 6.70 ± 0.7a | 5.8 ± 0.3a | 4.31 ± 0.6b | 6.36 ± 1.0a | 5.7 ± 0.4a | 4.79 ± 0.3b | 7.96 ± 0.4c | 6.96 ± 0.4a |
| 20:5n-3 | 0.23 ± 0.1a | 0.57 ± 0.1b | 0.72 ± 0.2b | 0.21 ± 0.1a | 0.49 ± 0.1b | 0.61 ± 0.2b | ND | ND |
| 22:5n-3 | 1.20 ± 0.1a | 1.46 ± 0.2a | 1.52 ± 0.3a | 1.04 ± 0.2a | 1.22 ± 0.2a | 1.38 ± 0.1a | 2.2 ± 0.2b | 1.20 ± 0.2a |
| 22:6n-3 | 6.5 ± 0.3a | 7.74 ± 0.5b | 9.73 ± 0.8c | 6.42 ± 0.4a | 7.25 ± 0.8b | 9.34 ± 0.7c | 5.04 ± 0.4d | 7.94 ± 0.4b |
| Heart microsomes | ||||||||
| SFA | 38.6 ± 4.1a | 37.4 ± 3.2a | 37.6 ± 3.6a | 37.3 ± 2.7a | 36.4 ± 3.3a | 35.9 ± 3.7a | 36.4 ± 1.9a | 37.9 ± 1.9a |
| MUFA | 21.8 ± 1.8a | 22.6 ± 2.2a | 20.8 ± 2.0a | 22.1 ± 3.1a | 21.1 ± 1.4a | 20.6 ± 2.3a | 19.6 ± 1.7a | 24.6 ± 3.7b |
| 18:2n-6 | 18.6 ± 1.6a | 17.1 ± 1.4a,b | 15.40 ± 1.3b | 19.7 ± 1.4a | 16.9 ± 1.6a,b | 15.9 ± 0.9b | 22.8 ± 1.2d | 14.8 ± 1.2b |
| 20:4n-6 | 13.2 ± 1.2a,b | 11.1 ± 1.1b,c | 9.5 ± 0.9c | 13.9 ± 1.3a,b | 11.5 ± 1.1b,c | 9.9 ± 0.7c | 14.2 ± 1.1a | 10.2 ± 1.1b,c |
| 20:5n-3 | 1.17 ± 0.1a | 2.35 ± 0.2b | 3.01 ± 0.3c | 1.25 ± 0.2a | 2.45 ± 0.1b | 2.91 ± 0.2c | ND | ND |
| 22:5n-3 | 1.21 ± 0.1a | 1.25 ± 0.2a | 1.33 ± 0.2a | 1.08 ± 0.1a | 1.2 ± 0.2a | 1.36 ± 0.1a | 0.76 ± 0.1b | 1.16 ± 0.2a |
| 22:6n-3 | 2.80 ± 0.3a | 4.51 ± 0.2b | 6.04 ± 0.5c | 2.70 ± 0.3a | 4.20 ± 0.4b | 5.80 ± 0.6c | 2.01 ± 0.1d | 4.81 ± 0.2b |
FO-LAF fish oil recovered by lactic acid fermentation; FO–EH fish oil recovered by enzymatic hydrolysis; GNO groundnut oil; 1.25, 2.50. 5.0 – percentage of EPA + DHA in diet; SFA - saturated fatty acids, MUFA – monounsaturated fatty acids, ND – not detected Data represent the mean ± SD, values not sharing common superscript within the column are significantly different (p < 0.05)
Cholesterol/phospholipid in microsomes of liver, heart and brain
The cholesterol level in liver, brain and heart microsomes was lowered by 7.5–22.5, 5.1–17.0 and 7.6–21.8 % respectively, in rats fed with FO-LAF as EPA + DHA compared to control group (Table 5). Similarly, decrease in cholesterol level was also observed in microsomes of liver (7.1–21.1 %), brain (6.5–15.7 %) and heart (6.8–20.8 %) of rats fed with FO-EH compared to control group (Table 5). There was significant (p < 0.05) reduction in liver, brain and heart microsomal cholesterol/phospholipid ratio at 5 % EPA + DHA fed group, compared to control. The change in cholesterol/phospholipid ratio was similar in microsomes of different tissues of CLO fed group and FVW-FO fed groups.
Table 5.
Cholesterol and cholesterol/phospholipid content in liver, brain and heart of rats fed with n-3 fatty acids recovered from fish visceral waste through biotechnological approaches at incremental levels
| Groups | Liver | Brain | Heart | |||
|---|---|---|---|---|---|---|
| TC | TC/PL | TC | TC/PL | TC | TC/PL | |
| FO-LAF-1.25 | 45.6 ± 2.1a,b | 0.202 ± 0.017a | 101.8 ± 4.1a,b | 0.239 ± 0.011a,b | 59.4 ± 2.6a,b | 0.284 ± 0.016a,b |
| FO-LAF-2.50 | 42.3 ± 2.7b,c | 0.189 ± 0.010a,c | 96.3 ± 3.0b,c | 0.221 ± 0.010b,c | 55.1 ± 2.1b,c | 0.261 ± 0.011b,c |
| FO-LAF-5.0 | 38.2 ± 1.7c | 0.172 ± 0.008c | 89.1 ± 4.3c | 0.208 ± 0.008c | 50.3 ± 2.9c | 0.249 ± 0.015c |
| FO-EH-1.25 | 46.1 ± 2.6a,b | 0.206 ± 0.006a | 100.3 ± 3.6a,b | 0.241 ± 0.010a,b | 59.9 ± 2.2a,b | 0.285 ± 0.017a,b |
| FO-EH-2.50 | 42.8 ± 2.0b,c | 0.193 ± 0.011a,c | 97.5 ± 3.7b,c | 0.220 ± 0.014b,c | 54.7 ± 3.1b,c | 0.256 ± 0.010b,c |
| FO-EH -5.0 | 38.9 ± 2.2c | 0.177 ± 0.013c | 90.5 ± 4.6c | 0.211 ± 0.012c | 50.9 ± 2.4c | 0.240 ± 0.021c |
| CLO-2.5 | 41.9 ± 2.0b,c | 0.190 ± 0.010a,c | 96.1 ± 3.2b,c | 0.218 ± 0.011b,c | 53.8 ± 3.3b,c | 0.252 ± 0.017b,c |
| GNO | 49.3 ± 2.3a | 0.216 ± 0.019a | 107.3 ± 3.4a | 0.247 ± 0.013a | 64.3 ± 2.8a | 0.305 ± 0.012a |
TC total cholesterol; PL phospholipid; FO-LAF fish oil recovered by lactic acid fermentation; FO–EH fish oil recovered by enzymatic hydrolysis; GNO groundnut oil; 1.25, 2.50. 5.0 – percentage of EPA + DHA in diet; Data represent the mean ± SD, values not sharing common superscript within the column are significantly different (p < 0.05)
Discussion
FO is the major source of PUFA especially n-3 fatty acids (EPA and DHA), which has significant effect on biochemical and physiological changes in the body. Higher levels of PUFA in tissue increase the susceptibility to oxidation and, as a result, may increase lipid peroxides concentration in the cellular systems (Shireen et al. 2008). Under such conditions, the endogenous antioxidant defense molecule should scavenge the superoxide and peroxides before they react with metal catalyst, which leads to oxidative stress. A decrease in activity of antioxidant enzymes may predispose cells to free radical damage (Haung and Fwu, 1993). Hence in the present study bioefficacy of FVW-FO recovered by FO-LAF and FO-EH was examined with respect to antioxidant potential and was compared to cod liver oil.
Feeding diet incorporated with FVW-FO at 1.25, 2.5 and 5.0 % of EPA + DHA resulted in elevation of the levels of lipid peroxides in tissues. Further the antioxidative defenses (CAT and SOD activity) increased significantly; this compensates the peroxides level in different tissues. The increased activity of the CAT may indicate the effective means of scavanging H2O2 that may be generated in the cells of different tissues. Earlier reports have shown that n-3 PUFA can elevate the mRNA expression of CAT and strengthens the antioxidant status in mice (Venkatraman et al. 1994; Aguilera et al. 2003). The inability of the antioxidant enzymes to prevent against oxidative damage in tissues may affect the cellular function such as membrane permeability. In this context, plasma membrane fluidity and activity of enzymes can get affected because lipid peroxidation rigidifies the membrane by extensive cross-linking of the membrane constituents. Levin et al. (1990) have proposed that the oxidation of membrane lipids results in the formation of peroxidation degradation products (Malondialdehyde), which leads to the crosslinking reactions of the lipid-lipid and lipid-protein type thereby making the membrane more rigid and hence less fluid.
Apart from oxidative stress, fatty acid composition of the membrane also affects the activity of membrane bound enzymes. The Na+K+ ATPase is an ubiquitous membrane-bound enzyme complex that plays a fundamental role in cellular function. Na+K+ ATPase activity have been shown to decrease in rats brain fed with n-3 PUFA deficient diet (Gerbi et al. 1999), which is also proven in long term deficiency of n-3 PUFA at optimal ATP concentrations (Bourre et al. 1984). Supplementation of fish oil in diet normalized the synaptosomal Na+ ATPase activity (Horrocks and Farooqui 2004), which may be due to stabilization of neural membranes by DHA. Feeding of DHA has shown to increase membrane fluidity and activity of Na+K+ ATPase (Hashimoto et al. 2001). Supplementation of CLO and DHA has been reported to increase acetylcholinesterase and Na+K+ ATPase activities in different parts of the brain (Kumosani and Moselhy 2011). Researchers have suggested that, the altered learning and memory abilities in rats and mice fed low LNA diet may be due to lower Na+K+ ATPase activity which may be a biochemical basis for the altered brain functions (Carrie et al. 1999, 2000). In the present study, we observed a significant increase in Na+K+ ATPase activity in liver and brain microsomes and Ca+Mg+ ATPases in heart microsomes of rats fed with diet containing 5 % EPA + DHA compared to control. The increase in the activity of acetylcholine esterase was also found with the increase in DHA levels in microsomes. This indicates that these enzymes show higher activity on feeding FO recovered from fishery byproducts indicating maintanance of membrane function.
PUFA and cholesterol levels in membrane also play important role in proper functioning of membrane proteins. Many studies using PUFA deficient diets have shown reductions in the level of DHA in brain and loss of many cognitive functions (Ahmed et al. 2002; Greiner et al. 1999). DHA level in liver, brain and heart microsomes increased significantly with incremental level of EPA + DHA in the diet from FO-LAF and FO-EH. It is also evident from the results that there was a marked increase in the proportion of total n-3 fatty acids (mainly 20:5 and 22:6), and a concomitant decrease in n-6 (20:4 and 18:2) fatty acids in the microsomes of rats fed on FVW-FO. As a consequence, there is a significant reduction in the ratio n-6: n-3 in fish oil fed groups compared to control. High cholesterol to phospholipid ratio decreases membrane fluidity, as the cholesterol sterically prevents the large motion of phospholipid fatty acyl chains (Yeagle et al. 1990). In young animals oxidative stress can raise the level of brain cholesterol to the level of aged rats (Denisova et al. 2001). Feeding rats with diet containing incremental levels of EPA + DHA from FVW-FO showed reduction in cholesterol level and cholesterol/phospholipid ratio in microsomes of different tissues suggesting the beneficial effect of FVW-FO. The present results, supports the view that feeding FVW-FO affects activities of antioxidant and membrane bound enzymes and microsomal lipid profile in a beneficial manner similar to CLO suggesting that FVW-FO can be a sustainable alternative to fish oil.
Conclusions
It is possible to utilize FVW-FO through solvent free biotechnological approaches as an alternative to commercial FO. This also increases the EPA + DHA and decreases the cholesterol store in the tissue at sub-cellular level similar to CLO. Although, lipid peroxidation increased to certain extent in different tissues, the increase in antioxidant enzymes may counter the effect of lipid peroxidation. Fish oil recovered from FVW was found to modulate the membrane bound enzymes in brain and liver. In addition, utilization of these processing wastes for the production of valuable biofunctional products can reduce the mounting economic values of FO and benefit the industry by minimizing the environmental pollution problems.
Acknowledgments
Authors thank Director of the institute for encouragement and permission to publish the work. AKR thanks Council of Scientific and Industrial Research, New Delhi for the award of Senior Research Fellowship for his doctoral program.
Conflict of interest
The authors declare that there are no conflicts of interest.
Abbreviations
- FVW
Fish visceral waste
- FO
Fish oil
- LAF
lactic acid fermentation
- EH
Enzymatic hydrolysis
- EPA
Eicosapentaenoic acid
- DHA
Docosahexaenoic acid
- CLO
Cod liver oil
- GNO
Ground nut oil
- FO-LAF
Fish oil recovered by lactic acid fermentation
- FO-EH
Fish oil recovered by enzymatic hydrolysis
- PUFA
Polyunsaturated fatty acids
- CAT
Catalase
- SOD
Superoxide dismutase
- GST
Glutathione transferase
References
- Aebi H. Catalase in vitro. In: Packer L, editor. Oxygen radicals in biological systems. Methods Enzymol. Orlando: Academic Press Inc.; 1984. [Google Scholar]
- Aguilera CM, Mesa MD, Ramirez TMC, Quiles JL, Gil A. Virgin olive and fish oils enhance the hepatic antioxidant defence system in atherogenic rabbits. Clin Nutr. 2003;22:379–382. doi: 10.1016/S0261-5614(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Moriguchi T, Salem N., Jr Decrease in neuron size in DHA deficient brain. Ped Neurol. 2002;26:210–218. doi: 10.1016/S0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. In: Neufeld EF, Ginsburg V, editors. Complex carbohydrates. Methods Enzymol. New York: Academic Press Inc; 1966. [Google Scholar]
- Amit KR, Jini R, Swapna HC, Sachindra NM, Bhaskar N, Baskaran V. Application of native lactic acid bacteria (LAB) for fermentative recovery of lipids and proteins from fish processing wastes: Bioactivities of fermentation products. J Aquatic Food Product Technol. 2011;20:32–44. doi: 10.1080/10498850.2010.528174. [DOI] [Google Scholar]
- Amit KR, Swapna HC, Bhaskar N, Baskaran V. Potential of seafood industry by-products as a source of recoverable lipids: Fatty acid composition of meat and non meat component of selected Indian marine fishes. J Food Biochem. 2012;36:441–448. doi: 10.1111/j.1745-4514.2011.00549.x. [DOI] [Google Scholar]
- Amit KR, Bhaskar N, Baskaran V. Bioefficacy of EPA-DHA from lipids recovered from fish processing wastes through biotechnological approaches. Food Chem. 2013;136:80–86. doi: 10.1016/j.foodchem.2012.07.103. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Pascal G, Durand G, Masson M, Dumont O, Piciotti M. Alterations in the fatty acid composition of rat brain cells (neurons, astrocytes and oligodendrocytes) and of subcellular fractions (myelin and synaptosomes) induced by a diet devoid of n-3 fatty acids. J Neurochem. 1984;43:342–348. doi: 10.1111/j.1471-4159.1984.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Carrie I, Clement M, De Javel D, Frances H, Bourre JM. Learning deficits in first generation OF1 mice deficient in (n-3) polyunsaturated fatty acids do not result from visual alteration. Neurosci Lett. 1999;266:69–72. doi: 10.1016/S0304-3940(99)00265-7. [DOI] [PubMed] [Google Scholar]
- Carrie I, Guesnet P, Bourre JM, Frances H. Diets containing long-chain n-3 polyunsaturated fatty acids affect behavior differently during development than ageing in mice. British J Nutr. 2000;83:439–447. [PubMed] [Google Scholar]
- Christon RA. Mechanism of action of dietary fatty acids in regulating the activation of vascular endothelial cells during atherogenosis. Nutr Rev. 2003;61:272–279. doi: 10.1301/nr.2003.aug.272-279. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71:2135–2235. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- Denisova NA, Cantuti-Castelvetri I, Hassan WN, Paulson KE, Joseph JA. Role of membrane lipids in regulation of vulnerability to oxidative stress in PC12 cells: implication for aging. Free Rad Biol Med. 2001;30:671–678. doi: 10.1016/S0891-5849(00)00513-X. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholine esterase activity. Biochem Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Flobe L, Oting F. Superoxide dismutase assay. In: Parker L, editor. Oxygen radicals in biological systems. Methods Enzymol. 105. Orlando: Acedemic press; 1984. pp. 93–104. [Google Scholar]
- Folch J, Lee M, Sloane SGH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gerbi A, Zrouga M, Maixent JM, Debray M, Durand G, Bourre JM. Diet deficient in alpha-linolenic acid alters fatty acid composition and enzymatic properties of Na +, K-ATPase isoenzymes of brain membranes in the adult rat. J Nutr Biochem. 1999;10:230–236. doi: 10.1016/S0955-2863(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Gluthenberg C, Alin P, Mannervik B. Glutathione transferase from rat testis. In: Meister A, editor. Glutamate, glutamine, glutathione and related compounds. Methods in Enzymology. Orlando: Academic Press Inc; 1985. [DOI] [PubMed] [Google Scholar]
- Greiner RS, Moriguchi T, Hutton A, Slotnick BM, Salem N., Jr Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory based and spatial learning task. Lipids. 1999;34:S239–S243. doi: 10.1007/BF02562305. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain MS, Yamasaki H, Yazawa K, Masumura S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids. 2001;34:1297–1304. doi: 10.1007/s11745-999-0481-6. [DOI] [PubMed] [Google Scholar]
- Haung CJ, Fwu ML. Degree of protein deficiency effects the extent of the depression of the antioxidant enzymes activities and the enhancement of tissue lipid peroxidation in rats. J Nutr. 1993;123:803–810. doi: 10.1093/jn/123.5.803. [DOI] [PubMed] [Google Scholar]
- Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. PLEFA. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Kaplay SS. Erythrocyte membrane Na+ and K+ activated adenosine triphosphatase in CM. J Clin Nutr. 1978;31:579–584. doi: 10.1093/ajcn/31.4.579. [DOI] [PubMed] [Google Scholar]
- Kumosani TA, Moselhy SS. Modulatory effect of cod-liver oil on Na-K ATPase in rats’ brain. Hum Exp Toxicol. 2011;30:267–274. doi: 10.1177/0960327110371699. [DOI] [PubMed] [Google Scholar]
- Levin G, Cogan U, Mokady S. Riboflavin deficiency and the function and fluidity of rat erythrocyte membranes. J Nutr. 1990;120:857–861. doi: 10.1093/jn/120.8.857. [DOI] [PubMed] [Google Scholar]
- Morrison MR, Smith M. Preparation of fatty acid methyl esters and dimethylacetyls from lipids with boron fluoride methanol. J Lipids Res. 1963;5:600–608. [PubMed] [Google Scholar]
- Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ramaprasad TR, Baskaran V, Krishnakanth TP, Lokesh BR. Modulation of antioxidant enzyme activities, platelets aggregation and serum prostaglandins in rats fed spray driednmilk containing n-3 fatty acids. Mol Cel Biol. 2005;277:19–26. doi: 10.1007/s11010-005-7094-x. [DOI] [PubMed] [Google Scholar]
- Reena MB, Lokesh BR. Effect of feeding blended and interesterified vegetables oil on antioxidant enzymes in rats. Food Chem Toxicol. 2011;49:136–143. doi: 10.1016/j.fct.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Rustad T. Utilization of marine by-products. Electron J Environ Agric Food Chem. 2003;2:458–463. [Google Scholar]
- Sandhir R, Julka D, Gill KD. Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol. 1994;74:66–71. doi: 10.1111/j.1600-0773.1994.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Shireen KF, Pace RD, Mahboob M, Khan AT. Effect of dietary vitamin E, C and soyabean oil supplementation on antioxidant enzyme activities in liver and muscle of rats. Food Chem Toxicol. 2008;46:3290–3294. doi: 10.1016/j.fct.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Statsoft . Statistica for Windows. Tulsa: Statsoft Inc.; 1999. [Google Scholar]
- Stewart JCM. Colorimetric estimation of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Swapna HC, Bijinu B, Amit KR, Bhaskar N. Simultaneous recovery of lipids and proteins by enzymatic hydrolysis of fish industry waste using different commercial proteases. App Biochem Biotechnol. 2011;164:115–124. doi: 10.1007/s12010-010-9119-5. [DOI] [PubMed] [Google Scholar]
- Turchini GM, Torstensen BE, Ng WK. Fish oil replacement in finfish nutrition. Rev Aquacul. 2009;1:10–57. doi: 10.1111/j.1753-5131.2008.01001.x. [DOI] [Google Scholar]
- Venkatraman JT, Chandrashekhar B, Kim JD, Fernandes G. Effects of n-3 and n-6 fatty acids on the activation and expression of hepatic antioxidant enzymes in autoimmune prone NZBxNZW F1 mice. Lipids. 1994;29:561–568. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- Yeagle PL, Albert AD, Boesze BK, Young J, Frye J. Cholesterol dynamics in membranes. J Biophysics. 1990;57:413–424. doi: 10.1016/S0006-3495(90)82558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda G, Shamir YK. Effect of urea sodium and calcium on microsomal ATPase activity in different parts of the kidney. Biochem Biophys Acta. 1971;233:133–136. doi: 10.1016/0005-2736(71)90365-8. [DOI] [PubMed] [Google Scholar]