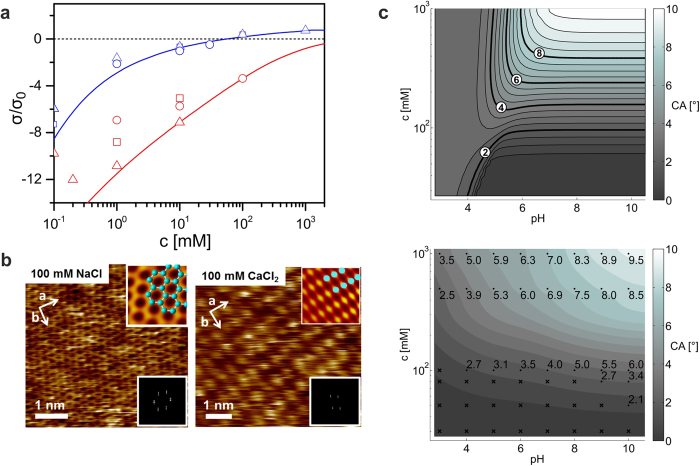
Figure 3. Ion adsorption at mica-water interface.
(a) Surface Charge calculated from ζ potential measurements (circles) vs. concentration of solutions of NaCl (red) and CaCl2 (blue) at pH 6. Solid lines: surface complexation model predictions. Blue triangles: AFM data from25; blue squares24, red triangles23, red squares32: surface forces apparatus measurements. The charge density is normalized by the characteristic scale 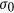 arising from the Poisson-Boltzmann equation,
arising from the Poisson-Boltzmann equation, 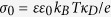 , where
, where 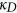 is the Debye parameter. (b) AFM images of mica-water interface showing the characteristic hexagonal lattice of mica in 100 mM NaCl solution (left), and a rectangular symmetry caused by (presumably hydrated) adsorbed Ca2+ ions in 100 mM CaCl2 (right). Insets: filtered zoomed views with overlaid lattice structure (top) and Fast Fourier Transform image of the same data (bottom). c Gray scale encoded contact angle vs. pH and CaCl2 concentration. Top: model prediction; bottom: experimental data. Symbols (x: θ < 2°) and numbers: experimental data same as Fig. 1b with interpolated gray scale. Smoothed lines are guides to the eye based on the experimental datapoints.
is the Debye parameter. (b) AFM images of mica-water interface showing the characteristic hexagonal lattice of mica in 100 mM NaCl solution (left), and a rectangular symmetry caused by (presumably hydrated) adsorbed Ca2+ ions in 100 mM CaCl2 (right). Insets: filtered zoomed views with overlaid lattice structure (top) and Fast Fourier Transform image of the same data (bottom). c Gray scale encoded contact angle vs. pH and CaCl2 concentration. Top: model prediction; bottom: experimental data. Symbols (x: θ < 2°) and numbers: experimental data same as Fig. 1b with interpolated gray scale. Smoothed lines are guides to the eye based on the experimental datapoints.