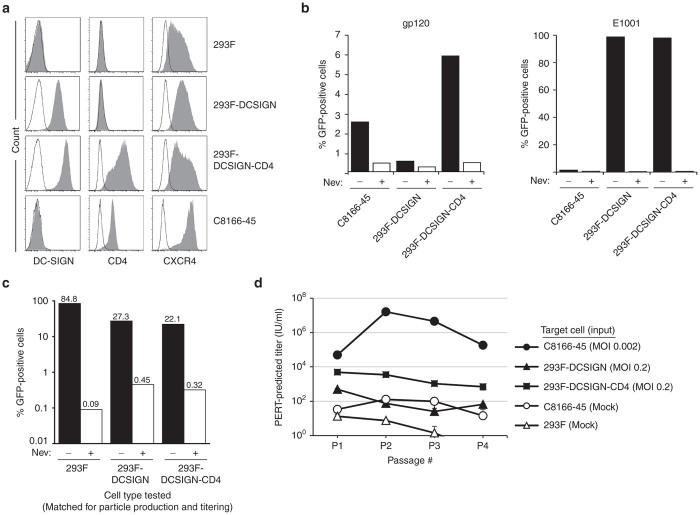
Figure 4.
Evaluation of a replication-competent lentivirus assay format utilizing a wild-type HIV-1 positive control virus and 293F-DCSIGN-CD4 cells (Approach B). (a) 293F, 293F-DCSIGN, 293F-DCSIGN-CD4, and C8166-45 cells were analyzed by flow cytometry for expression of DC-SIGN, the HIV-1 receptor CD4, and coreceptor CXCR4. Open histograms indicate unstained controls. (b) Transduction by gp120- or E1001-enveloped vector was assessed in the following cell lines: C8166-45, 293F-DCSIGN, and 293F-DCSIGN-CD4. Cells were incubated with vector encoding GFP in the presence or absence of the reverse-transcriptase inhibitor nevirapine (Nev). At 48 hours post-transduction, cells were analyzed for GFP fluorescence. (c) E1001-enveloped vector production was assessed in 293F, 293F-DCSIGN, and 293F-DCSIGN-CD4 cells. Using calcium phosphate, each cell type was transfected with vector component plasmids to generate E1001-enveloped HIV-1 vector encoding GFP. At 48 hours post-transfection, harvested supernatant was incubated with fresh cognate cells in the presence or absence of nevirapine; GFP fluorescence was analyzed after 72 hours. (d) C8166-45, 293F-DCSIGN, or 293F-DCSIGN-CD4 cells were inoculated with wild-type HIV-1 (strain NL4-3) at the MOI noted. Subsequent release of viral particles into supernatant fluid was measured using the F-PERT assay over four passages. As a negative control, spent media from mock-infected parental C8166-45 or 293F cells was assayed in parallel at each passage. F-PERT, fluorescent-product enhanced reverse transcriptase; GFP, green fluorescence protein.