Figure 7.
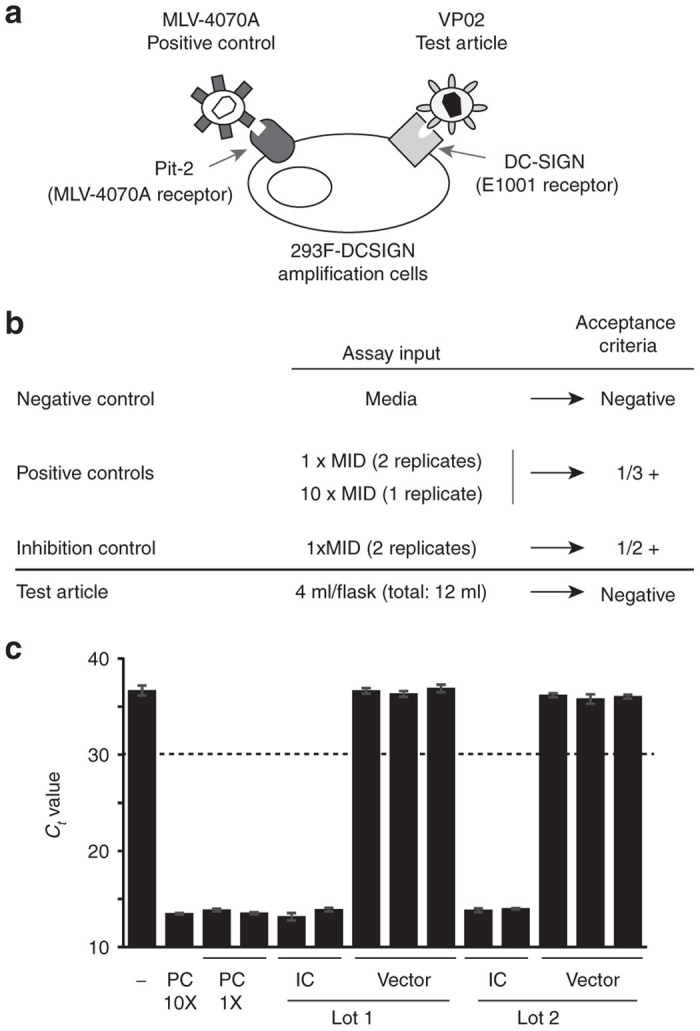
Replication-competent lentivirus (RCL) testing of large-scale vector production lots. (a) Schematic of the final design for an RCL assay specific for VP02 vector and EOPC. The assay positive control virus consists of MLV-4070A and the amplification cell line is 293F modified to express DC-SIGN (293F-DCSIGN), the receptor for the E1001 envelope glycoprotein present on the surface of VP02. (b) Controls and acceptance criteria for the VP02-specific RCL assay. The positive controls consist of MLV-4070A in cell culture media, whereas the inhibition control consists of vector test article spiked with MLV-4070A. At least one of the positive controls and one of the inhibition controls must be positive for the assay to be valid. (c) Test results for two of six independent production lots of VP02 clinical drug substance. A 12-ml sample of each bulk drug substance was split equally into three T-flasks. Two additional flasks contained an identical amount of vector, in addition to the MLV-4070A positive control spike (positive controls, PC; inhibition controls, IC). F-PERT analysis was performed on cell supernatants after six passages. Each bar represents a separate flask; error bars represent standard deviation from the mean for F-PERT replicates. Dashed line represents cut-off for assay positivity; ≥ 2 F-PERT replicates must return a Ct value of ≤30 in order for a flask to be deemed positive. F-PERT, fluorescent-product enhanced reverse transcriptase; MLV, murine leukemia virus.
