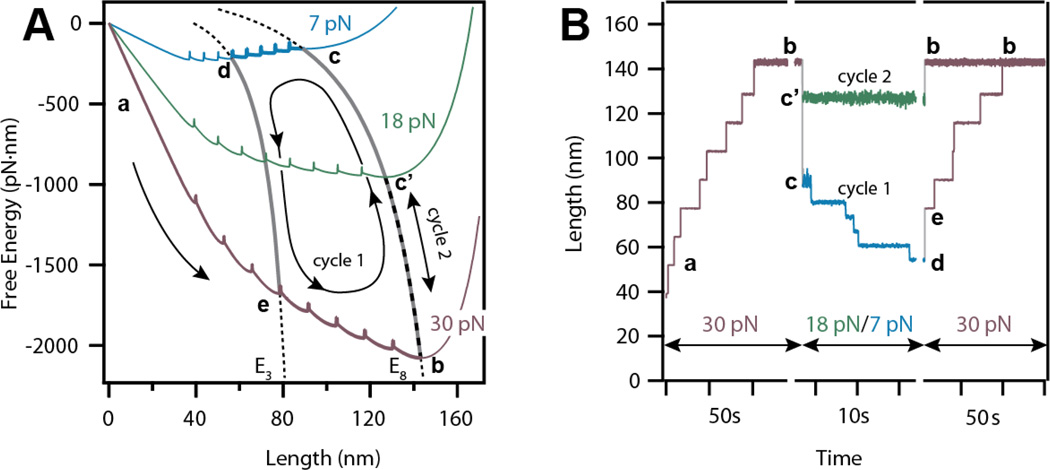
Figure 3. Force-protocols to explore the free energy landscape of a tandem protein using Brownian dynamics.
A) Free energies and E-curves depicting the trajectory of a single unfolding-refolding-unfolding Brownian Dynamics simulation. Movement along a single free energy may result in folding or unfolding while diffusion along an E-curve changes the end-to-end length of the protein without folding events. B) Traces obtained from Brownian dynamics simulations for two different cycles (cycle 1 and 2). The first cycle considers the total unfolding at 30 pN from a to b, followed by a quench of 7 pN causing diffusion along the E8 curve to c, and a final refolding of five domains from c to d. For the second cycle, the refolding force is reduced to 18 pN instead of 7 pN, causing the shortening of the polyprotein from b to c’ without refolding any domains.