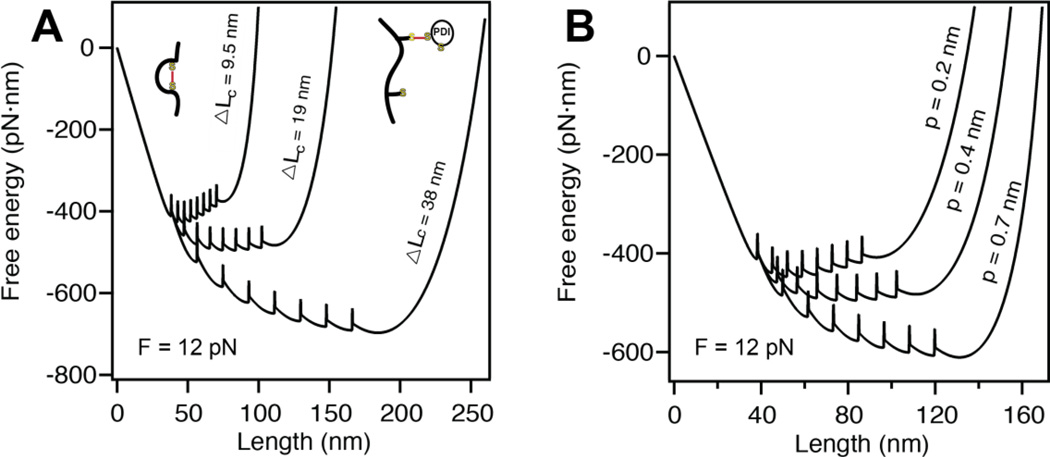
Figure 4. Altering the parameters of the entropic elasticity affects protein folding.
A) Decreasing the contour length of our polyprotein from ΔLc = 38 nm to ΔLc = 9.5 nm, shifts the global minimum free energy from favoring the completely unfolded state to the completely folded state at a constant force of F =12 pN. Hence, the rate of folding is considerably increased by the decreasing ΔLc. B) A similar effect is captured by altering the persistence length at a constant force of F = 12 pN. Increasing the stiffness of the polymer from p = 0.2 nm to p = 0.7 nm also shifts the minimum free energy from fully folded to fully unfolded.