Abstract
Significant disparities exist between genders for the development and progression of several gastro-intestinal (GI) diseases including cancer. Differences in incidence between men vs women for colon, gastric and hepatocellular cancers suggest a role for steroid sex hormones in regulation of GI carcinogenesis. Involvement of intrinsic gender-linked mechanisms is also possible for esophageal adenocarcinoma as its incidence is disproportionally high among men. However, the cause of the observed gender differences and the potential role of androgens in esophageal carcinogenesis remains unclear, even though the cancer-promoting role of androgen receptors (AR) shown in other cancers such as prostate and bladder suggests this aspect warrants exploration. Several studies have demonstrated expression of ARs in esophageal cancer. However, only one study has suggested a potential link between AR signaling and outcome - poorer prognosis. Two groups have analyzed data from cohorts with prostate cancer and one of these found a decreased incidence of esophageal squamous and adenocarcinoma after androgen deprivation therapy. However, very limited information is available about the effects of androgen and AR-initiated signaling on esophageal cancer cell growth in vitro and in vivo. Possible mechanisms for androgens/AR involvement in the regulation of esophageal cancer growth are considered, and the potential use of AR as a prognostic factor and clinical target is highlighted, although insufficient evidence is available to support clinical trials of novel therapies. As esophageal adenocarcinoma is a gender linked cancer with a large male predominance further studies are warranted to clarify the role of androgens and ARs in shaping intracellular signaling and genomic responses in esophageal cancer.
Keywords: Esophageal cancer, Androgens, Androgen receptor
Core tip: Esophageal cancers, especially adenocarcinoma, are gender-linked malignancies, with a male predominance. Previous studies have demonstrated expression of androgen receptors in both adenocarcinoma and squamous cell esophageal cancer. However, the impact of androgens in development and progression of these cancers is unclear. Androgen-deprivation therapy has not been explored, even though it is successfully used in treatment of prostate cancers. Further studies are warranted to clarify the role of androgens and androgen receptors in shaping intracellular signaling and genomic responses in esophageal cancer.
WHY ANDROGEN RECEPTORS?
Gender associated differences in the incidence of various gastrointestinal (GI) cancers are well recognized, with some diseases dominated by the female gender, including gallstone disease and primary biliary cirrhosis, and others by the male gender, including gastroesophageal reflux disease (GERD) and colon cancer[1-4]. Esophageal cancers of both major subtypes show a male predilection, occurring 3-4 times more commonly in men than women globally[5]. This is most marked for esophageal adenocarcinoma (EAC), with reported sex ratios (M:F) ranging from 5:1 in France, 6:1 in Australia and Sweden, 8:1 in the United States to 10:1 in the United Kingdom[6-8]. An analysis of the Surveillance, Epidemiology and End Results (SEER) Registry in the United States confirmed a significant rise in EAC among males and a slower rise in females from 1973 to 2008, with an overall M:F ratio of 7.66[8]. This gender bias towards men is out of proportion to the gender distribution for the underlying risk factors, predominantly obesity and GERD[9]. For the other major subtype, esophageal squamous cell cancer (ESCC), a male propensity also occurs, but is less marked than in EAC, and it appears to occur concomitantly with male dominance of the main risk factors, smoking and alcohol consumption[10]. Reasons for the disproportionate rise in EAC in men have not been investigated in detail, but some studies implicate sex hormones as contributing factors[11].
Sex steroid hormones, predominantly estrogens in women and androgens in men, demonstrate gender specific concentration profiles. These hormones regulate cell growth and behavior via a variety of estrogen and androgen receptor subtypes which are distributed widely throughout normal and abnormal human tissues, including cancers. The role of sex hormones in the development of prostate and breast cancers is well known[12,13]. Estrogen receptors (ER) have been shown to have a role in esophageal adenocarcinomas[14,15]. Androgen receptors (AR) are also widely expressed in human tissues and have been identified in esophageal cancer[16-20]. Higher circulating levels of testosterone and dihydrotestosterone (DHT) have been identified in patients who develop Barrett’s esophagus, the precursor lesion for EAC, after controlling for age, body mass index (BMI), and GERD symptom profiles[21]. Analysis of the SEER database has shown that patients with a previous diagnosis of prostate cancer are less likely to develop EAC, raising the possibility that the androgen deprivation therapy used for prostate cancer may reduce the risk of EAC[22]. This study also found that the incidence of ESCC was lower in individuals with a previous diagnosis of prostate cancer and suggested that lifestyle modifications could be an additional factor. Nevertheless, the presence of ARs in EAC and the association between testosterone and Barrett’s esophagus suggest that androgens might play a role in the development of EAC, and would provide a logical explanation for the male dominance of the disease[21].
However, this hypothesis is contradicted by findings in overweight or obese individuals who develop esophageal adenocarcinoma. As obesity has been unequivocally shown to be a major risk factor for the development of esophageal adenocarcinoma, consideration must also be given to the lower level of circulating androgens in obese individuals[9,23]. Testosterone is converted into estrogens in intra-abdominal adipose tissues by aromatase, and this might further decrease local concentration of androgens. Hence, local conversion of androgens to estrogens might contribute to the development of EAC, and provide an explanation for an interaction between obesity, androgens, and male gender in the genesis of EAC.
CIRCULATING ANDROGENS
Testosterone, dihydrotestosterone (DHT), androstenedione, and dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) comprise the group of circulating human androgens which prevail in men. These are 19-carbon steroid molecules synthesized from cholesterol in the adrenal cortex, testes (in men) and ovaries (in women). Androgens can be converted into 18-carbon estrogens by aromatase enzymes predominantly in the liver, but also in gonadal, adipose and other tissues. An estimated 5% of the serum testosterone produced in men is catalyzed to the more potent androgen DHT by 5α reductase enzymes. AR affinity of DHT is 3-fold greater than the affinity of testosterone and 15- to 30-fold greater than adrenal androgens affinity[24]. High AR affinity of DHT makes this androgen the most potent hormone activator of AR-regulated transcription. The more abundant but less potent adrenal steroids DHEA and DHEAS, are precursors for intracellular production of more active androgens, and also estrogens under 5α reductase and aromatase activity[25].
The vast majority of testosterone circulates bound to plasma proteins: 40%-50% of testosterone is bound to albumin, 50%-60% is strongly bound to sex hormone-binding globulin (SHBG), with only 1%-2% being free[26]. Both free testosterone and albumin-bound testosterone are bioavailable. However, they show some differences in biological effects[27]. For instance, free testosterone and DHT act via ARs in the cell nucleus and regulate AR-dependent gene transcription. Protein bound circulating androgens, however, are biologically active by a different signaling pathway. Testosterone and DHT bound to SHBG has been shown to act via a specific cytoplasmic G-protein coupled SHBG receptor in prostate cancer cells, rather than by binding nuclear AR[28]. Thus, the free circulating androgens are a potent minority and act via nuclear AR, whereas SHBG-bound testosterone and DHT may exert effects by cytoplasmic SHBG receptors.
The levels of all androgens increase at puberty and peak during adolescence, then gradually decrease with age[29]. Alterations in the ratios of circulating androgens followed by depletion of sex steroid hormones are important consequences of normal aging and are associated with vulnerability to disease in hormone-responsive tissues[30]. The significant decline in male sex steroids, and its clinical consequences, has been dubbed androgen deficiency in aging males (ADAM)[30]. In contrast to menopause in females, androgen deficiency in aging males is rarely accompanied by the loss of reproductive function. Androgen deficiency in aging males associated hormonal changes are gradual, with bioavailable testosterone levels declining 2%-3% annually from approximately 30 years of age[30,31].
In women, testosterone is produced primarily through peripheral conversion of androstendione (50%). Testosterone is also produced in the ovaries (25%) and the adrenal glands (25%)[32]. During pregnancy, the placenta may also serve as a source of the testosterone[33]. There is at least a 10-fold difference in testosterone levels between males and females of reproductive age. The plasma concentration of the hormone in males is between 10 to 35 nmol/L and in females is 0.7-2 nmol/L[34]. This contrast adds support to the hypothesis that estrogens might be protective against development of gastrointestinal cancers, but androgens may facilitate carcinogenesis[15,35]. This hypothesis is compatible with epidemiological data, which show a decline in male dominance in older age groups, i.e., postmenopausal females. The SEER dataset has shown a peak gender ratio of 11:1 in favor of males in the 50-54 years age bracket, but falling to 4:1 in the 75-79 year age group[8].
ANDROGEN RECEPTOR SIGNALING
Androgens regulate protein synthesis and influence tissue remodeling via the AR, which along with other steroid hormone receptors are members of the nuclear receptor superfamily[36] (Figure 1). As the AR gene is located on the X chromosome (Xq11.2) it exists as a single copy in males. The AR protein is a ligand-inducible zinc finger transcription factor that regulates target gene expression[37]. Androgen receptors possess four structurally and functionally distinct domains: the NH2-terminal transactivation domain, the DNA-binding domain, a hinge region and the COOH-terminal ligand-binding domain[37]. Two AR isoforms have been found: the predominant isoform B (110 kDa) and the less dominant and shorter isoform A (80 kDa)[38]. Novel AR splice variants designated AR3, AR4 and AR5 in androgen-insensitive prostate cancer cell lines have recently been described[39].
Figure 1.
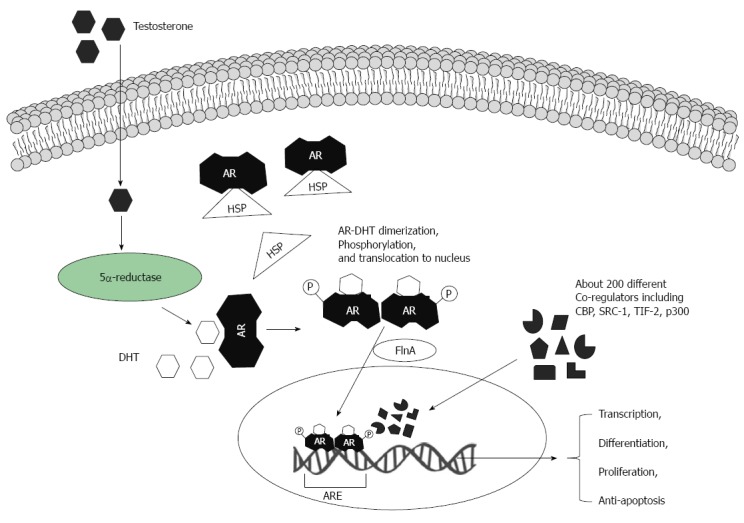
Biological actions of androgens via androgen receptors. Testosterone molecules translocate via the plasma membrane and are transformed into dihydrotestosterone (DHT) by 5α-reductase. The androgen receptor (AR) is located in cytoplasm and bound to heat shock protein (HSP). DHT binds to the AR and HSP is then released. Ligand-AR complexes can be phosphorylated (and/or are modified by other post-translational mechanisms). Two ligand-AR complexes form homodimers and move into the nucleus. AR nuclear translocation is facilitated by filamin A (FlnA). In the cell nucleus ligand-AR complexes bind to specific DNA elements - androgen-responsive elements (ARE), which are in target gene promoters. These regulate target gene expression at the transcriptional level. A large variety of co-factors and regulators can orchestrate AR-induced gene transcription.
Non-ligand-bound AR is located in the cytoplasm where it associates with heat shock proteins (HSPs). Ligand binding induces a conformational change in the AR, resulting in release of heat-shock proteins, dimerization, phosphorylation, and translocation of the complex to the nucleus (Figure 1). Microtubule proteins, specifically filamin A (FlnA) help to transport ligand-AR complexes to the cell nucleus[40]. Homo-dimerized AR interacts with a constellation of transcriptional co-regulators and transcription factors leading to specific transcriptional activation or repression of target genes[41]. AR-inducible genes coordinate the development of male sexual characteristics (both anatomical and mental), spermatogenesis, and maintain skeletal homeostasis[42].
NONGENOMIC AR SIGNALING PATHWAY
Extranuclear or non-genomic androgen effects are distinct from androgen genomic actions. Extranuclear effects can be detected within in the first seconds or minutes after the application of androgen to cells or tissues[43,44]. Nongenomic androgen activity involves rapid induction of second messenger signal transduction cascades, including calcium and cAMP fluxes, activation of protein kinase A(PKA), protein kinase C (PKC), and mitogen-activated protein (MAP) kinases[45] (Figure 2). These dynamic changes in protein kinase activity shape a specific phosphorylation profile that, in turn, transfers new signals to the cell nucleus to switch on genes[46].
Figure 2.
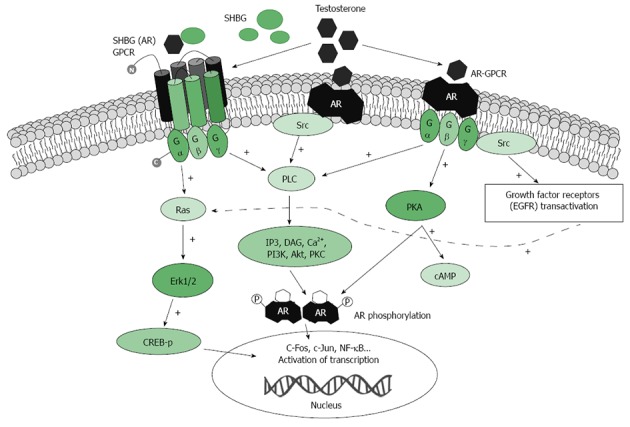
Schematic presentation of testosterone-activated extra-nuclear signaling pathways. Testosterone binds to undefined membrane-associated androgen receptor(s) (mAR) that might transduce signaling downstream to phospholipase c (PLC). Activation of PLC produces several second messengers including Ins(1,4,5)P3 (IP3) and DAG. Ca2+ influx then leads to an increase in intracellular Ca2+. Alternatively, in the ERK pathway, Testosterone binds to the membrane-associated receptor, which associates with and activates Src kinase. In a third proposed mechanism SHBG-AR GPCR activates Ras, which in turn activates the cascade of phosphorylation. The ERK pathway phosphorylates CREB to modulate gene expression. AR: Membrane associated androgen receptor; CREB: cAMP response element binding protein; DAG: Diacylglycerol; GPCR: G-protein-coupled receptor; IP3: Inositol trisphosphate; PLC: Phospholipase C; SRC: Src kinase; RAS: RasGTPase protein; +: Indicates positive effect on activation.
Extranuclear androgen signaling is not only faster than genomic signaling, but might occur through cytoplasmic receptors that are different to the traditional nuclear AR. Recent reports confirm that opposite androgen and AR-mediated effects in prostate cancers might depend not only on cell type and tumor stage, but also on AR type and localization[47]. Several membrane-localized receptor targets for androgens have been proposed[37,48]. Androgens have been shown to rapidly stimulate PKA and induce production of cAMP via binding to G-protein coupled membrane receptor for the SHBG-testosterone complex[28,37].
It has also been suggested that testosterone might bind a membrane-associated receptor (mAR) which might be distinct from the classical nuclear AR. Nuclear-localized ARs lack a standard transmembrane structure and hydrophobicity, and thus it is likely that AR should undergo significant posttranslational modification[49], and/or interact with other proteins that might facilitate AR anchorage to the membranes. Supporting this, Akt1 and lipid raft association with mAR has recently been demonstrated in prostate cancer cells[50]. Several groups have reported specific binding of testosterone to the plasma membrane in different cell types[48,51]. Although mAR has not yet been purified or cloned, using sub-cellular fractionation and immunohistochemistry techniques, membrane association of classical ARs has been shown in Xenopus oocytes, in Chinese Hamster Ovary cells, Sertoli cells, and T cells[49,51-53]. Importantly, mAR activation has been shown to induce profound apoptosis of prostate cancer cells in vitro and in vivo in mouse xenografts, with suppression not only of cell growth, but also metastatic motility of cancer cells[48]. Activation of these mARs appears also to initiate apoptotic pathways in colon cancers, even in the absence of intracellular AR[54].
Data from these studies indicate that functional mARs trigger strong anti-tumorigenic effects, suggesting mAR as a novel target for the development of selective cancer treatments[55]. However, it remains unclear whether mARs are also expressed in other tumors, including esophageal cancers, and also whether activation of mAR could induce anti-tumorigenic effects similar to those seen in prostate cancer.
ANDROGEN RECEPTORS AS A THERAPEUTIC TARGET
Since Huggins and Hodges[56] demonstrated the effectiveness of surgical castration in men with prostate cancer in 1941, the role of androgens and AR signaling in the carcinogenesis of prostate cancers has been under investigation. Chemical castration with anti-androgen therapy for prostate cancer is standard clinical practice, and has led to substantial gains in survival[57]. Intriguingly, higher serum testosterone levels are not associated with higher rates of prostate cancer. It appears that the prostate is saturated at low levels of circulating androgens and that neither low or high serum testosterone levels are associated with a decrease or increase in the risk of this cancer[58]. When considering promotion of prostate cancer growth, the AR is sensitive to basal levels of androgens, and only complete elimination of circulating androgens is effective treatment. In a recent population study a reduced risk of developing esophageal cancer was shown in subset of 343538 patients with prostate cancer who were exposed to androgen deprivation therapy, supporting the involvement of androgens in the development and/or growth of esophageal malignancies[22].
Given the shared embryological origin of prostate and urogenital epithelium, it was also recently proposed that AR signaling might contribute to bladder cancer[59]. This is supported by evidence in murine models that bladder cancer is not induced in AR knockout mice compared with controls[60]. However, clinical studies associate the loss of AR with more advanced stage, indicating that ARs might not be a good therapeutic target[61].
The role of ARs has also been investigated in breast cancer. Emerging evidence suggests that androgen signaling pathways may exert inhibitory effects on the growth of normal mammary epithelial cells and thus play a protective role in the pathogenesis of breast cancer[62]. In vitro studies have demonstrated that androgens may counteract the proliferative effect of estrogens in AR-positive breast cancer cells[63]. Up-regulation of AR expression or treatment with AR agonists markedly decreases ER-alpha transcriptional activity[63]. However, the utility of AR ligand therapy has yet to be verified clinically.
While prostate and breast tissue develop and function under direct control of steroid hormones, gastrointestinal tissues are not traditionally considered to be targets of steroid hormones. The mechanisms of AR functioning in these tissues are largely unknown. The expression of ARs in normal and cancerous gastrointestinal tissues also remains controversial. Nevertheless, colonic tissues have been shown to express the majority of functional steroid hormone receptors[54,64]. In a clinical study conducted a decade ago, ARs were expressed in all samples from 35 patients with colon adenocarcinoma, with similar expression in both neoplastic and non-neoplastic surrounding mucosa. However, binding activity for AR exhibited in cancer specimens differed from non-neoplastic colonic mucosa[65]. Subsequent studies have shown specific AR genotypes to be associated with colorectal cancers[66].
In vivo studies also support a role for ARs in colon cancer. In 1983 Izbicki et al[67,68] reported that the administration of androgens promoted colon cancer tumorigenesis in rats, and a year later that the steroid hormones induced tumor growth remission in a mouse xenograft model. More recently, it has been suggested that enhanced susceptibility of male mice to intestinal tumor growth derives from the classical nuclear AR, while in contrast membrane-localized mAR has anti-tumorigenic effects independent of classical AR signaling[56]. mAR consistently induces tumor regression reduced invasiveness, and potent pro-apoptotic responses, supporting potential targeting of mAR for treatment of colon cancer[69].
A few studies have attempted to define the role of ARs in gastric cancers[70-72]. Gan and co-authors evaluated 60 pairs of fresh gastric cancers with matched normal gastric mucosa from patients undergoing gastrectomy[71]. Samples were processed to determine levels of AR mRNA and proteins. Unlike the typical nuclear expression seen in breast and prostate cancer cells, the ARs in the gastric cancers were localized mostly in cytoplasm. Also, the protein level of AR receptors in the gastric cancers was significantly lower than that expressed in normal gastric mucosa, but positive AR expression correlated with a better prognosis[71,72]. However, the authors ultimately concluded that the functional significance of ARs in gastric cancer appeared to be limited.
ANDROGEN RECEPTORS IN ESOPHAGEAL SQUAMOUS CELL CARCINOMA
In 1985, Kobayashi[73] investigated the effects of sex hormones on the development of ESCC in a rat model. The highest incidence of ESCC was in male rats, followed by female rats treated with testosterone. This fell to 0% in castrated rats treated with estradiol. Female rats with no hormonal manipulation also had low rates (8%). The authors concluded that ESCC growth is inhibited by estrogen and stimulated by androgens. However, the expression of ARs was not addressed in this study.
AR expression in ESCC is summarized in Table 1. Matsuoka et al[74] were the first to detect ARs in a cell line (KSE-1) derived from a male esophageal squamous cell cancer. The KSE-1 cell line had a binding content of 4.2 fmol/mg protein for the ER and 2.2 fmol/mg of protein for the AR in the cytoplasm. Proliferation of this cell line was suppressed by estrogen and accelerated by testosterone. Ueo et al[75] characterized ARs and conducted treatment experiments in two ESCC cell lines, KSE-1 and KSE-2. Similar to Matsuoka et al[74], the proliferation of KSE-1 was increased by dihydrotestosterone and decreased by estradiol, although sex hormones had no impact on the growth of KSE-2. Receptor analysis found KSE-1 to be positive for both AR and ER, but KSE-2 was negative for both. In a mouse xenograft model, KSE-1 tumor growth was suppressed by estradiol, whereas no effect was seen in KSE-2. Interestingly, no growth-promoting effect was seen with dihydrotestosterone in either cell line in vivo, which is consistent with the concept of testosterone saturation in the context of prostate cancer[58]. Tanaka et al[76] further investigated KSE-1, and found that the tumor growth stimulation that occurs with AR activation is mediated by fibroblast growth factor 8 (FGF-8) signaling known as androgen-induced growth factor.
Table 1.
Androgen receptor expression in esophageal cancer
| Ref. | No. of patients | Male:female | Histological type | AR | Conclusion |
| Matsuoka et al[74], 1987 | NA | NA | SCC cell line | 2.2 fmol/mg | Proliferation of KSE-1 cell line is inhibited by estrogen and enhanced by testosterone |
| Tihan et al[19], 2001 | 25 | 21:4 | AC (n = 11)SCC (n = 14) | 7 males and 1females, 5 EAC and 3 SCC were positive | Presence of AR in human esophageal cancer is an impetus for further studies to assess anti-androgen therapy for treatment and or prevention of these tumors. |
| Yang et al[20], 2001 | 31 | 26:5 | SCC | Cancer tissues: 40.56 ± 18.19 fmol/mg, normal tissues: 7.84 ± 3.21 fmol/mg | AR and estrogen receptors have close relationship with the biologic behavior and prognosis of esophageal SCC |
| Tiffin et al[80], 2003 | 20 | 10:10 | AC (ND)BE (ND) | Very weakly positive in 1 male with EAC and 1 female with BE | Androgen receptors are not implicated in BE or AC |
| Awan et al[17], 2007 | 23 | 20:3 | AC (n = 18)SCC (n = 5) | 16 samples (EAC = 13, SCC = 3) showed positive nuclear staining. AC occurred in 12 males and 1 female, while in SCC 2 males and 1 female | AR expressed in the stroma of esophageal AC may induce paracrine effects following stimulation by androgens (including tumor-derived), possibly via FGFs |
| Nordenstedt, et al[18], 2012 | 30 | 20:10 | BE (n = 10)Controls (n = 20) | All BE cases were negative. Only 1 male control was found AR expressed in squamous esophageal mucosa | AR were negative in BE warrants further research to find alternative explanations for the male predominance in BE and EAC |
AR: Androgen receptor; EAC: Esophageal adenocarcinoma; SCC: Squamous cell cancer; BE: Barrett’s esophagus; NA: Not applicable; ND: Not described.
Yamashita et al[77] identified ARs in ESCC specimens from 21 patients. Two tumors were successfully xenografted into male nude mice and cultured as cell lines, and the growth of the cell lines in the presence of sex hormones was assessed. Similar to the other studies, Testosterone stimulated growth, whereas estrogen had no impact. Tihan et al[19] used immunohistochemistry to determine AR expression in ESCC resection specimens. Positive staining for AR was found in 3 of 14 (21%) specimens. No differences in survival were found with respect to AR status, but the study size was small. Yang et al[20] detected ARs and ERs in ESCC specimens from 31 patients (26 male, 5 female) using a radio-ligand binding assay method. Compared with the normal esophageal tissues, more ARs were detected in the ESCC tissues (40.56 ± 18.19 fmol/mg vs 7.84 ± 3.21 fmol/mg). The expression of AR correlated with gender, tumor differentiation, invasion, and the lymph node metastasis status, but not age of the patient or tumor location[20]. The authors concluded that the expression of AR impacts on the biological behavior and prognosis of ESCC[19,20].
Dietzsch et al[78] (2003) investigated androgen pathways in ESCC arising in South African males by targeting AR mutations which were known to occur in prostate cancer. As in prostate cancer, known AR mutations were associated with increased susceptibility to the disease. These authors concluded that this may, in combination with sequencing for other known mutations, be useful in developing a test for genetic susceptibility to the cancer in order to target appropriate investigations. It also suggests that the mutation in question [a short (GGC)n allele] increases AR activity, which would explain the increased incidence of this cancer in men with this mutation, and also implicates AR signaling in the pathogenesis of the disease, at least in some patient populations.
ANDROGEN RECEPTORS IN ESOPHAGEAL ADENOCARCINOMA
Understanding whether androgens influence the development of esophageal cancers is important, as intervention before progression to malignancy offers the possibility of prevention. Barrett’s esophagus is recognized to be the precursor for EAC, and offers an opportunity for prevention or early intervention[79]. Case-control studies have tested associations between serum sex steroid hormones and the development of Barrett’s esophagus in men, and a strong positive association with free testosterone has been demonstrated[21,79]. This relationship appears to be independent of age or BMI suggesting a direct relationship between testosterone and the progression of Barrett’s esophagus to EAC. Contradicting this, however, Nordenstedt et al[18] failed to identify ARs in esophageal mucosal biopsies from 10 men with Barrett’s esophagus vs ARs seen in squamous epithelium from only 1 of 20 controls (10 males and 10 females). They concluded that the absence of ARs in Barrett’s esophagus meant that alternative explanations for the male predominance in Barrett’s esophagus and EAC should be sought. However, these findings were based on Barrett’s esophagus tissues rather than EAC, and the sample size was small.
The first clinical study addressing the role of androgens and AR in EAC was reported by Lagergren and Nyren[35] (1998). This was a population-based, retrospective cohort study of 100215 individuals, including patients diagnosed with prostate cancer and receiving anti-androgen therapy (mainly in the form of estrogen analogues), which attempted to identify whether this treatment altered the risk of EAC. However, analysis by latency intervals after prostate cancer diagnosis revealed no clear trend toward increasing or decreasing risk of esophageal cancer over a 4 year time period[35]. This study did not support either the hypothesis of a tumor-promoting role for androgens, or a protective effect for estrogens. However, the levels of AR expression or serum androgens before and after treatment were not assessed in this study, and the study duration was relatively short. Furthermore, since estrogens were used as anti-androgen therapy, the study actually investigated mixed effects of two different signaling systems rather than AR blockade or deprivation specifically. A more recent study was reported by Cooper and Trudgill[22], who this time identified a reduced risk of developing EAC in the subset of prostate cancer patients undergoing androgen deprivation therapy. This later study suggests further investigation towards potential use of anti-androgens or androgen-deprivation approaches in esophageal cancers should be considered.
Tiffin et al[80] analyzed 20 paraffin-embedded specimens of EAC arising in Barrett’s esophagus from 10 male and 10 female patients. Both the EAC and Barrett’s esophagus regions of the resection specimens were examined. Weak staining for AR was only identified in EAC in one male, and in the Barrett’s esophagus segment in one female patient. Mild to moderate staining for estrogen receptors was identified in two EACs from men and in six EACs from women, leading the authors to conclude that androgen pathways were not responsible for gender inequalities in this disease, but that evaluation of ER signaling may be valuable. However, this contrasts with the findings of Tihan et al[19] (2001) in which 5 of 11 (45%) EAC specimens were assessed by immunohistochemistry to be AR positive.
Awan et al[17] determined AR expression in 18 patients with EAC and 5 with ESCC, with areas of both normal squamous epithelium and cancer examined. Thirteen EAC and three ESCC specimens showed positive stromal staining for AR, but no staining was seen in the cancer epithelium. In normal squamous epithelium from the same patients, ARs were expressed in 5 patients with EAC and 2 with ESCC. Interestingly, fasting serum testosterone values in male patients were significantly higher in patients with EAC than age-matched controls (median 18.2 nmol/L vs 12.5 nmol/L). These levels declined significantly following surgical removal of the cancer, suggesting that paracrine effects of androgens may be implicated in the pathogenesis of the disease[17]. In keeping with the findings of Tanaka et al[76] (2001), FGF-8b expression was common in cancer in males but absent in cancer in females and in normal mucosa, further supporting the theory that this is an important growth factor in men.
Paradox of obesity and androgen conversion in esophageal cancers
Androgen as a risk factor for the development of esophageal cancer, in particular EAC, appears at odds with evidence that obesity is a major risk factor for EAC, as obese individuals have lower levels of circulating androgens[7,9,23]. This paradox might be explained by the endocrine actions of visceral fat in males. Male pattern obesity is dominated by the expansion of visceral fat in the abdominal area, which contrasts to predominantly subcutaneous fat deposition in women. Visceral adipose tissue has been shown to act as an endocrine organ and so produces and secretes a wide range of biologically active molecules, including hormones and cytokines[81]. Adipose tissue pathogenicity markedly differs between visceral and subcutaneous fat location. Visceral fat is a highly metabolic tissue marked by higher production of TNFalpha, plasminogen activator inhibitor 1 (PAI1), IL6, and C-reactive protein (CRP), while producing lower amounts of the anti-inflammatory adipokine adiponectin, which correlates more strongly with subcutaneous fat[82-85].
While the accumulation of adipose tissue in abdominal area (visceral fat) is supported by androgens, increased aromatase activity and conversion of androgens to estrogen has been shown to occur in adipose tissues of aging and obese men. Furthermore, adipocytes can secrete estrogens in proportion to total fat mass, and influence physiological processes through paracrine and autocrine actions[85,86]. Active synthesis of estrogens is associated with increased plasma leptin, that, in turn, can stimulate a further reduction of androgen level[30]. In adipocytes, activation of aromatase is self-induced by pro-inflammatory cytokines such as TNF-α which is secreted locally by adipocytes and infiltrating macrophages, and secures conversion of testosterone to estrogen[82,87]. Locally synthesized estrogens may influence cancer cell growth[15]. It is also possible that estrogens may alter the sensitivity of AR to testosterone and thereby impact upon EAC growth, and this might represent a possible therapeutic target in esophageal cancers similar to what has been found in prostate cancers[22,37].
Supporting this, adipose-tissue secreted cytokines and hormones have been shown to exert local paracrine actions in the tissues in which they are formed and on surrounding neighbor cells[81,82,85]. Metabolic conversion of androgen precursors to estrogen and further estrogen metabolites has been observed in several normal tissue and tumors, facilitated by expression of the hormone converting enzyme aromatase as reviewed in detail elsewhere[88]. Notably, the observation that aromatase is often overrepresented in adipose tissue has not yet been verified in visceral fat proximal to esophagus in men.
It has been suggested that increased aromatase activity and conversion of testosterone to estrogens are activated as a feed-back mechanism which regulates adipocyte numbers. This is supported by findings in an aromatase knockout mice where estrogen plays an inhibitory role during adipogenesis to limit adipocyte number[89]. It is possible that in aromatase activation an increased production of estrogen metabolites might result in ineffective, and potentially reversed action of the hormones in aging and obese men, although no work has been reported which addresses this possibility. The potential role of estrogen signaling in esophageal cancers has been recently reviewed, suggesting a role in the genesis of EAC[15]. It remains, however, to confirm that adipose tissue located in close proximity to the esophagus can actively transform androgens to estrogens and influence proliferation of ER-positive esophageal tumors. More detailed investigation of steroid hormone metabolism in adipose tissue located in close proximity to the esophagus is warranted, and an opportunity exists to evaluate abdominal adipose (visceral) tissue samples from individuals with EAC and Barrett’s esophagus to see if increased aromatase expression and active conversion of androgens to estrogens is evident.
CONCLUSION
While epidemiological evidence for the male propensity for esophageal cancer is clear, especially the dominance of the male gender in EAC, data evaluating the role of male sex steroids and the androgen receptor in the pathogenesis of this cancer is more difficult to dissect apart. In vitro evidence from cell culture work and subsequent testing in murine models suggests a significant influence of sex hormones upon cancer growth, and that this effect is consistent with expression patterns of the receptors. However, the identification of ARs in human tissue specimens has been less straightforward, with expression reported by some authors, but not by others. While androgens and their receptors probably do play a role in the carcinogenesis of esophageal cancer, more work is required to evaluate this role and to understand their contribution to the genesis of esophageal cancer. Although therapeutic implications and novel therapies are desirable, there is currently insufficient evidence to support a clinical trial of androgen deprivation therapy in this cancer. Further investigation of sex hormone signaling pathways in esophageal cancer appears justified, and in the future this might lead to improved understanding of the genesis of esophageal cancer or even new treatments.
Footnotes
Conflict-of-interest: The authors declare no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 24, 2015
First decision: March 10, 2015
Article in press: April 17, 2015
P- Reviewer: Chen XL, Dobrucali AM, Ganguly E, Triadafilopoulos G S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Borum ML. Hepatobiliary diseases in women. Med Clin North Am. 1998;82:51–75. doi: 10.1016/s0025-7125(05)70594-0. [DOI] [PubMed] [Google Scholar]
- 2.Richter JE. Gastroesophageal reflux disease and its complication. In: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease., editor. 8th ed. Philadelphia: Saunders Elsevier; 2006. pp. 905–936. [Google Scholar]
- 3.Tung BY, Kowdley KV. In: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 8th ed. Philadelphia: Saunders Elsevier; 2006. pp. 1461–1476. [Google Scholar]
- 4.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Lepage C, Drouillard A, Jouve JL, Faivre J. Epidemiology and risk factors for oesophageal adenocarcinoma. Dig Liver Dis. 2013;45:625–629. doi: 10.1016/j.dld.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu LN, Kanarek NF, Tsai HL, Rudin CM, Brock MV. Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973-2008) Dis Esophagus. 2014;27:757–763. doi: 10.1111/dote.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, Webb PM, Green AC. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 10.Lindblad M, Ye W, Lindgren A, Lagergren J. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg. 2006;243:479–485. doi: 10.1097/01.sla.0000205825.34452.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagergren K, Lagergren J, Brusselaers N. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: a systematic review and meta-analysis. Int J Cancer. 2014;135:2183–2190. doi: 10.1002/ijc.28869. [DOI] [PubMed] [Google Scholar]
- 12.Flüchter SH, Weiser R, Gamper C. The role of hormonal treatment in prostate cancer. Recent Results Cancer Res. 2007;175:211–237. doi: 10.1007/978-3-540-40901-4_13. [DOI] [PubMed] [Google Scholar]
- 13.Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108:351–361. doi: 10.1007/s10549-007-9618-4. [DOI] [PubMed] [Google Scholar]
- 14.Sukocheva OA, Wee C, Ansar A, Hussey DJ, Watson DI. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Dis Esophagus. 2013;26:628–635. doi: 10.1111/dote.12000. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Sukocheva OA, Hussey DJ, Watson DI. Estrogen, male dominance and esophageal adenocarcinoma: is there a link? World J Gastroenterol. 2012;18:393–400. doi: 10.3748/wjg.v18.i5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 17.Awan AK, Iftikhar SY, Morris TM, Clarke PA, Grabowska AM, Waraich N, Watson SA. Androgen receptors may act in a paracrine manner to regulate oesophageal adenocarcinoma growth. Eur J Surg Oncol. 2007;33:561–568. doi: 10.1016/j.ejso.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Nordenstedt H, Younes M, El-Serag HB. Expression of androgen receptors in Barrett esophagus. J Clin Gastroenterol. 2012;46:251–252. doi: 10.1097/MCG.0b013e318238353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tihan T, Harmon JW, Wan X, Younes Z, Nass P, Duncan KL, Duncan MD. Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res. 2001;21:3107–3114. [PubMed] [Google Scholar]
- 20.Yang LF, Ji XG, Xing MD, Sun M, Zhao HY. The relationship between esophageal cancer and androgen-estrogen receptors. Zhongguo Xiongxinxieguan Waike Linchuang Zazhi. 2001;8:154–156. [Google Scholar]
- 21.Cook MB, Wood SN, Cash BD, Young P, Acosta RD, Falk RT, Pfeiffer RM, Hu N, Su H, Wang L, et al. Association between circulating levels of sex steroid hormones and Barrett’s esophagus in men: a case-control analysis. Clin Gastroenterol Hepatol. 2015;13:673–682. doi: 10.1016/j.cgh.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper SC, Trudgill NJ. Subjects with prostate cancer are less likely to develop esophageal cancer: analysis of SEER 9 registries database. Cancer Causes Control. 2012;23:819–825. doi: 10.1007/s10552-012-9950-9. [DOI] [PubMed] [Google Scholar]
- 23.Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord. 1993;17:643–649. [PubMed] [Google Scholar]
- 24.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 25.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–221. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68. doi: 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 27.Cefalu WT, Pardridge WM, Chaudhuri G, Judd HL. Serum bioavailability and tissue metabolism of testosterone and estradiol in rat salivary gland. J Clin Endocrinol Metab. 1986;63:20–28. doi: 10.1210/jcem-63-1-20. [DOI] [PubMed] [Google Scholar]
- 28.Nakhla AM, Leonard J, Hryb DJ, Rosner W. Sex hormone-binding globulin receptor signal transduction proceeds via a G protein. Steroids. 1999;64:213–216. doi: 10.1016/s0039-128x(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 29.Giusti G, Gonnelli P, Borrelli D, Fiorelli G, Forti G, Pazzagli M, Serio M. Age-related secretion of androstenedione, testosterone and dihydrotestosterone by the human testis. Exp Gerontol. 1975;10:241–245. doi: 10.1016/0531-5565(75)90001-7. [DOI] [PubMed] [Google Scholar]
- 30.Morley JE. Androgens and aging. Maturitas. 2001;38:61–71; discussion 71-3. doi: 10.1016/s0378-5122(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 31.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 32.McKenna TJ, Fearon U, Clarke D, Cunningham SK. A critical review of the origin and control of adrenal androgens. Baillieres Clin Obstet Gynaecol. 1997;11:229–248. doi: 10.1016/s0950-3552(97)80035-1. [DOI] [PubMed] [Google Scholar]
- 33.Warshaw ML, Johnson DC, Khan I, Eckstein B, Gibori G. Placental secretion of androgens in the rat. Endocrinology. 1986;119:2642–2648. doi: 10.1210/endo-119-6-2642. [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab. 1986;15:259–278. doi: 10.1016/s0300-595x(86)80024-x. [DOI] [PubMed] [Google Scholar]
- 35.Lagergren J, Nyrén O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:913–915. [PubMed] [Google Scholar]
- 36.Carlberg C, Seuter S. Dynamics of nuclear receptor target gene regulation. Chromosoma. 2010;119:479–484. doi: 10.1007/s00412-010-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 38.Gao T, McPhaul MJ. Functional activities of the A and B forms of the human androgen receptor in response to androgen receptor agonists and antagonists. Mol Endocrinol. 1998;12:654–663. doi: 10.1210/mend.12.5.0112. [DOI] [PubMed] [Google Scholar]
- 39.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol Endocrinol. 2000;14:1618–1626. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- 41.Yadav N, Heemers HV. Androgen action in the prostate gland. Minerva Urol Nefrol. 2012;64:35–49. [PubMed] [Google Scholar]
- 42.Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. doi: 10.1146/annurev-physiol-030212-183656. [DOI] [PubMed] [Google Scholar]
- 43.Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003;144:3449–3455. doi: 10.1210/en.2003-0044. [DOI] [PubMed] [Google Scholar]
- 44.Lang F, Alevizopoulos K, Stournaras C. Targeting membrane androgen receptors in tumors. Expert Opin Ther Targets. 2013;17:951–963. doi: 10.1517/14728222.2013.806491. [DOI] [PubMed] [Google Scholar]
- 45.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 46.Michels G, Hoppe UC. Rapid actions of androgens. Front Neuroendocrinol. 2008;29:182–198. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Wen S, Niu Y, Lee SO, Chang C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat Rev. 2014;40:31–40. doi: 10.1016/j.ctrv.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kampa M, Kogia C, Theodoropoulos PA, Anezinis P, Charalampopoulos I, Papakonstanti EA, Stathopoulos EN, Hatzoglou A, Stournaras C, Gravanis A, et al. Activation of membrane androgen receptors potentiates the antiproliferative effects of paclitaxel on human prostate cancer cells. Mol Cancer Ther. 2006;5:1342–1351. doi: 10.1158/1535-7163.MCT-05-0527. [DOI] [PubMed] [Google Scholar]
- 49.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 50.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282:29584–29593. doi: 10.1074/jbc.M703310200. [DOI] [PubMed] [Google Scholar]
- 51.Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, Sekeris CE, Mossmann H. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 52.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci USA. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 54.Gu S, Papadopoulou N, Gehring EM, Nasir O, Dimas K, Bhavsar SK, Föller M, Alevizopoulos K, Lang F, Stournaras C. Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol Cancer. 2009;8:114. doi: 10.1186/1476-4598-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life. 2009;61:56–61. doi: 10.1002/iub.150. [DOI] [PubMed] [Google Scholar]
- 56.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 57.Hodgson MC, Bowden WA, Agoulnik IU. Androgen receptor footprint on the way to prostate cancer progression. World J Urol. 2012;30:279–285. doi: 10.1007/s00345-011-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65:115–123. doi: 10.1016/j.eururo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Izumi K, Miyamoto H. The role of the androgen receptor in the development and progression of bladder cancer. Jpn J Clin Oncol. 2012;42:569–577. doi: 10.1093/jjco/hys072. [DOI] [PubMed] [Google Scholar]
- 60.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 61.Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, Scherr DS. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64:383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 62.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–617. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 63.Garay JP, Park BH. Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012;2:434–445. [PMC free article] [PubMed] [Google Scholar]
- 64.D’Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008;65:1523–1543. doi: 10.1007/s00018-008-7552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berta L, Fronticelli Baldelli C, Fazzari A, Radice E, Bargoni A, Frairia R, Gaetini A. Sex steroid receptors, secondary bile acids and colorectal cancer. A possible mechanism of interaction. Panminerva Med. 2003;45:261–266. [PubMed] [Google Scholar]
- 66.Slattery ML, Sweeney C, Murtaugh M, Ma KN, Wolff RK, Potter JD, Caan BJ, Samowitz W. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2936–2942. doi: 10.1158/1055-9965.EPI-05-0514. [DOI] [PubMed] [Google Scholar]
- 67.Izbicki JR, Schmitz R, Kamran D, Izbicki W. Androgens as promoters of colon carcinogenesis. Cancer Detect Prev. 1983;6:355–362. [PubMed] [Google Scholar]
- 68.Izbicki JR, Schmitz R, Hoppen HO, Izbicki W, Troidl H. Effects of steroid hormone therapy on primarily xenotransplanted human colorectal adenocarcinomas. J Cancer Res Clin Oncol. 1984;108:345–350. doi: 10.1007/BF00390470. [DOI] [PubMed] [Google Scholar]
- 69.Gu S, Honisch S, Kounenidakis M, Alkahtani S, Alarifi S, Alevizopoulos K, Stournaras C, Lang F. Membrane androgen receptor down-regulates c-src-activity and beta-catenin transcription and triggers GSK-3beta-phosphorylation in colon tumor cells. Cell Physiol Biochem. 2014;34:1402–1412. doi: 10.1159/000366346. [DOI] [PubMed] [Google Scholar]
- 70.Tian Y, Wan H, Lin Y, Xie X, Li Z, Tan G. Androgen receptor may be responsible for gender disparity in gastric cancer. Med Hypotheses. 2013;80:672–674. doi: 10.1016/j.mehy.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Gan L, He J, Zhang X, Zhang YJ, Yu GZ, Chen Y, Pan J, Wang JJ, Wang X. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566. doi: 10.1186/1471-2407-12-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang BG, Du T, Zang MD, Chang Q, Fan ZY, Li JF, Yu BQ, Su LP, Li C, Yan C, et al. Androgen receptor promotes gastric cancer cell migration and invasion via AKT-phosphorylation dependent upregulation of matrix metalloproteinase 9. Oncotarget. 2014;5:10584–10595. doi: 10.18632/oncotarget.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi K. [Effect of sex hormone on the experimental induction of esophageal cancer] Nihon Geka Gakkai Zasshi. 1985;86:280–289. [PubMed] [Google Scholar]
- 74.Matsuoka H, Sugimachi K, Ueo H, Kuwano H, Nakano S, Nakayama M. Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma. Cancer Res. 1987;47:4134–4140. [PubMed] [Google Scholar]
- 75.Ueo H, Matsuoka H, Sugimachi K, Kuwano H, Mori M, Akiyoshi T. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50:7212–7215. [PubMed] [Google Scholar]
- 76.Tanaka S, Ueo H, Mafune K, Mori M, Wands JR, Sugimachi K. A novel isoform of human fibroblast growth factor 8 is induced by androgens and associated with progression of esophageal carcinoma. Dig Dis Sci. 2001;46:1016–1021. doi: 10.1023/a:1010753826788. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita Y, Hirai T, Mukaida H, Kawano K, Toge T, Niimoto M, Hattori T. Detection of androgen receptors in human esophageal cancer. Jpn J Surg. 1989;19:195–202. doi: 10.1007/BF02471585. [DOI] [PubMed] [Google Scholar]
- 78.Dietzsch E, Laubscher R, Parker MI. Esophageal cancer risk in relation to GGC and CAG trinucleotide repeat lengths in the androgen receptor gene. Int J Cancer. 2003;107:38–45. doi: 10.1002/ijc.11314. [DOI] [PubMed] [Google Scholar]
- 79.de Jonge PJ, van Blankenstein M, Grady WM, Kuipers EJ. Barrett’s oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiffin N, Suvarna SK, Trudgill NJ, Riley SA. Sex hormone receptor immunohistochemistry staining in Barrett’s oesophagus and adenocarcinoma. Histopathology. 2003;42:95–96. doi: 10.1046/j.1365-2559.2003.01513_3.x. [DOI] [PubMed] [Google Scholar]
- 81.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 82.Bertin E, Nguyen P, Guenounou M, Durlach V, Potron G, Leutenegger M. Plasma levels of tumor necrosis factor-alpha (TNF-alpha) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab. 2000;26:178–182. [PubMed] [Google Scholar]
- 83.Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 84.You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, Tylavsky FA, Harris TB, Kritchevsky SB. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alemany M. Steroid hormones interrelationships in the metabolic syndrome: an introduction to the ponderostat hypothesis. Hormones (Athens) 2012;11:272–289. doi: 10.14310/horm.2002.1356. [DOI] [PubMed] [Google Scholar]
- 86.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 87.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, Cleary MP. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 89.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]


