Abstract
AIM: To evaluate the qualitative and quantitative changes in N-linked glycosylation, which occurred in association with diethyl nitrosamine-induced hepatocellular carcinoma (HCC) in rodents.
METHODS: Liver tissues of (1) normal (non-tumor-bearing) rats; and (2) tumor-bearing rats; were collected and were used for histological and GlycanMap® analyses. Briefly, GlycanMap® analysis is a high-throughput assay that provides a structural and quantitative readout of protein-associated glycans using a unique, automated 96-well assay technology coupled to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and custom bioinformatics. Histopathological studies were carried out to ensure the development of HCC in the tested animals.
RESULTS: The N-glycomic analysis revealed 5 glycans; Glc1Man9GlcNAc2, Gal2Man3GlcNac4Fuc1Neu1, Man4GlcNac2, Gal2Man3GlcNac4Neu3OAc3, and Man3GlcNac5Fuc1, which showed significant changes in rat HCC tissues when compared with normal liver tissues. Four glycans were increased (P < 0.05) and Glc1Man9GlcNAc2 was decreased (5.89 ± 0.45 vs 3.54 ± 0.21, P < 0.01) in HCC tissues compared to normal liver tissues. An increase (66.5 ± 1.05 vs 62.7 ± 1.1, P < 0.05) in high-mannose structures in HCC rats was observed compared to normal rats. Importantly, HCC rats showed an increase (P < 0.05) in both tumor-associated carbohydrates and in branched glycans. The changes in glycans correlated well with glycan flow changes reported in the glycan biosynthetic pathway, which indicates the importance of enzyme activities involved in glycan synthesis at different subcellular localizations.
CONCLUSION: The reported HCC-associated changes in glycan flow and subcellular localization explain the increase in high mannose glycans and siayl Lewis glycans common in HCC liver tissues.
Keywords: Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, Hepatocellular carcinoma, Glycomics, Biosynthetic pathways
Core tip: Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide, yet it is still poorly diagnosed. Glycans are emerging as sensitive and simple biomarkers of various malignant diseases. Utilizing the cutting-edge N-glycomic analysis, we identified 5 glycans that were significantly different between normal and tumor-bearing rats. An increase in high-mannose structures in HCC rats was observed compared to normal rats. HCC rats showed an increase in both tumor-associated carbohydrates and branched glycans. The changes in glycans correlated with glycan flow changes reported in the glycan biosynthetic pathway, which indicates the importance of enzyme activities involved in glycan synthesis at different subcellular localizations.
INTRODUCTION
Glycosylation is an important post-transcriptional modification with over 50% of total proteins being glycosylated[1]. Glycosylation is crucial for cellular interaction; therefore, cancer involves noticeable changes in glycan biosynthesis. Thus, protein glycosylation (N- and O- glycosylation) is sensitive to environmental changes and is commonly disrupted in various diseases such as cancer[2-4]. Altered N-glycans and O-glycans may result from changes in the expression levels of glycan synthetic enzymes or from disruption of the biosynthetic pathway such as the ER-Golgi chain[5]. These changes can provide valuable insights into cancer development and its progression, and these altered glycosyl epitopes are classified as tumor-associated carbohydrate (TAC) antigens. Some of those TAC antigens are already in clinical use for the diagnosis and monitoring of cancers[6-9]. Recent technological advancements in the area of glycan isolation and characterization has turned glycomics into a new potential tool for cancer prognosis and treatment.
Since the beginning of the glycomics era, liver diseases such as fibrosis, cirrhosis and hepatocellular carcinoma (HCC) have been the prime targets in many investigations[10-13]. That trend was mainly driven by the lack of reliable diagnostic biomarkers of HCC. Many studies have shown that the serum N-glycan profile is a non-invasive marker for liver diseases such as HCC[12,14-17]. Serum N-linked glycoproteins are mainly produced in the liver and by B-lymphocytes, thus changes in the serum N-glycan profile reflect the physiology of liver and/or B-cells[12]. Various studies have shown significant changes in total N-glycan levels in the serum of HCC patients, thereby demonstrating the potential of glycans and glycome profiles as efficient biomarkers of HCC[18,19]. Alpha fetoprotein (AFP) is the only known biomarker used for HCC. AFP levels remain unchanged during the onset of HCC, which makes its application as a differential diagnostic marker of other liver diseases unreliable[20,21]. The glycosylated form of AFP, AFP-L3, was proposed as new tumor marker and was approved by the FDA in 2006, for the early detection of primary HCC[22]. Studies also showed an increase in the fucosylated glycans in HCC patients compared to patients with chronic liver diseases[23,24]. However, there was no such increase in fucosylation in the liver tissues of HCC patients when compared with normal tissues, signifying the importance of glycan analyses in cancer tissues along with serum[25]. The serum profile of diethyl nitrosamine (DEN)-induced HCC has been investigated for new biomarkers[26]. The present study focuses on the analysis of N-glycan changes in DEN-induced HCC liver tissues. The glycan profiles of liver lysates from tumor-bearing rats and normal rats were analyzed using the Ezose GlycanMap® platform to identify changes in the N-glycans of liver tissues in a HCC rodent model. Changes in N-glycan levels in the liver tissues could identify novel alterations in N-glycan profiles that are highly specific to HCC and its progression. A glycomic marker in rat livers may also be useful for screening drugs in this DEN-HCC rat model.
MATERIALS AND METHODS
Animal care and use statement
Adult male albino rats, Wistar strain, (150-200 g) were obtained from the Animal House, UAE University, UAE. They were maintained on a standard pellet diet and tap water ad libitum and were kept in polycarbonate cages with wood chip bedding under a 12 h light/dark cycle at room temperature (22 °C-24 °C). The rats were acclimatized to the environment for two-weeks prior to experimental use. This study was approved by the Animal Research Ethics Committee, UAE University.
Hepatocarcinogenesis model
Hepatocarcinogenesis was initiated by DEN and promoted by 2-Acetylaminofluorene (AAF) as described by Espandiari et al[27] and modified by Amin et al[28]. Briefly, as a mitotic proliferative stimuli, 4-d fasted rats were re-fed and the following day the rats were injected once intraperitoneally with DEN at 200 mg/kg b.wt., dissolved in saline. Two weeks post-DEN treatment, the rats received 6 daily intragastric doses of 2-AAF (30 mg/kg in 1% Tween 80) to promote liver cancer.
Treatment regime
The rats were divided into 2 groups (7 animals per group) as follows: Group 1 (normal): Rats were administered water at 5 mL/kg b.wt throughout the experimental period and were injected with one dose of saline. In Group 2 (tumor-bearing or HCC): Hepatocarcinogenesis was developed as detailed earlier. Group 1 was treated with an equal volume of vehicle. After 22 wk of DEN administration, all animals were anesthetized 24 h after the last treatment. Following anesthesia, liver samples were dissected out.
Histology and immunohistochemistry
Diethylether-anesthetized rats were sacrificed and the livers excised. Samples of the right, left and caudate liver lobes were immediately fixed in 10% buffered formalin for histopathological examination. The remaining liver was frozen in liquid nitrogen and stored at -80 °C. Histological sections were embedded in paraffin after being dehydrated in ethanol. Five-micrometer sections were mounted onto slides, stained with Hematoxylin and Eosin and then examined under an Olympus DP71-light microscope.
For the immunohistochemistry assay, mounted sections were immersed in sodium citrate buffer (0.1 mol/L, pH 6) and placed in a water bath for 15 min to unmask antigen epitopes. To prevent nonspecific binding to endogenous peroxidase, the sections were incubated with 0.3% H2O2 (Sigma Chemical Co., United States) in methanol. Anti-GST-p (Medical and Biological Laboratories Co., Tokyo, Japan) was incubated with the slides overnight at 4 °C. The slides were then washed with PBS and incubated with secondary antibody, polyvalent biotinylated goat-anti-rabbit antibody, for 10 min at room temperature (1:200 dilution). The universal LSAB kit and DAB plus substrate kit were both used to perform a standard staining protocol and additional counter-staining was performed using hematoxylin. Slides were mounted and observed under an optical microscope (Olympus DP71), and tissue micrographs were obtained. In individual samples, ten fields were randomly selected to quantify positive cells (400 ×). A color image processor was used to count GST-p foci for more than 15 cells.
Glycomic analysis
Sample preparation: Liver tissue was collected from normal and tumor-bearing rats. The liver tissues were lysed in 50 mmol/L Tris-acetate, pH 7.4, containing 1% sodium dodecyl sulfate, 5 mmol/L EDTA, and 0.15 mmol/L NaCl followed by homogenization with a Polytron homogenizer. Homogenates were centrifuged to extract soluble materials, and 1/10 vol of 20% Triton X-100 was added. The extracts were dialyzed against 20 mmol/L ammonium bicarbonate for 48 h at 4 °C. After the dialysis, the recovered solution was lyophilized using a SpeedVac concentrator. Residual materials were reconstituted in 50 mmol/L ammonium bicarbonate and frozen until used.
N-linked glycan isolation and purification: The protein concentrations were normalized to 1 mg/mL and each sample was analyzed in duplicate to quantitate N-linked glycans using Ezose Sciences’ proprietary GlycanMap® methodology reported by Nishimura, Furukawa and Miura[29-31]. Aliquots of each sample were spiked with internal standards to aid quantification. The aliquots were denatured and then trypsinized, followed by heat-inactivation. The N-glycans were then enzymatically released from the peptides by treating with PNGase F (New England Biolabs) and the released glycans were subjected to solid-phase processing using chemo-selective beads. After being captured on the beads, the sialic acid residues were methyl esterified to stabilize them in the mass spectrometer. The glycans were simultaneously released from the beads and labeled, and aliquots of the recovered materials were spotted onto a MALDI target plate. Utilizing a fully automated, 96-well format, robotic technology, steps from initial aliquoting to spotting on the MALDI plate were performed.
Mass spectrometric analysis: MALDI-TOF MS analysis was performed on an Ultraflex III mass spectrometer (Bruker Daltonics) in the positive-ion, reflectron mode using a proprietary matrix composition. From the bead-based processing step, samples were spotted in quadruplicate, and spectra were obtained in an automated manner using the AutoXecute feature in flexControl software (Bruker Daltonics). Mass spectra were analyzed using Ezose’s proprietary bioinformatics programs (Figure 1A).
Figure 1.
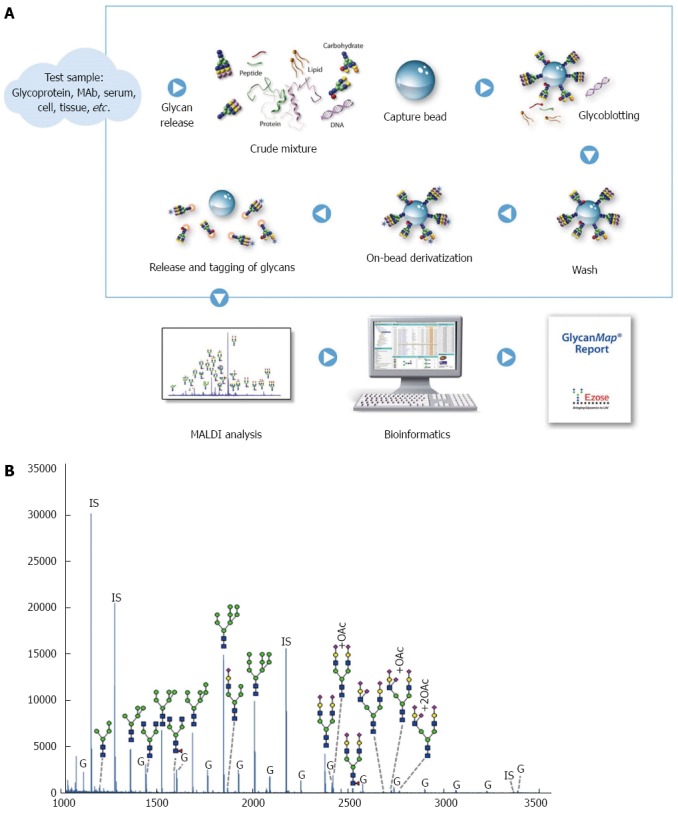
GlycanMap workflow. A: Workflow; B: Glycan profile in rat liver lysates. The spectrum was generated from sample N5 and is representative of the N-glycan profile in rat liver lysates. Taking advantage of the symbol nomenclature of the Consortium for Functional Glycomics, selected peaks were labeled with the proposed glycan structures. All spectra were collected on a MALDI-TOF mass spectrometer in reflection mode, which yields high mass accuracy and isotopic resolution for more accurate glycan identification. Glycans were quantified by comparing the peak intensity of each glycan-derived peak to those of the internal standards (IS). The liver lysates also contained varying amounts of glycogen (G).
Quantification using internal standard: Raw mass spectra were smoothed and baseline subtracted prior to peak detection. Glycan compositions were assigned to each peak based on m/z. The intensities were normalized to that of an internal standard with known concentration. The normalized intensities from each of the quadruplicate spectra were averaged. Data were also corrected for the inherent decrease in peak intensity as m/z increases in mass spectrometric measurements, yielding absolute concentrations (in nmol/μg protein) that are equivalent to those created by HPLC/fluorescent glycan analysis methods. Glycan amounts were calculated by comparing peak heights to those of the internal standards. Glycan amounts were compared between treatment groups.
Statistical analysis
Data was analyzed by GraphPad Prism 6. Analysis of differences between the means of the tumor-bearing and control groups was performed using the Student’s t-test.
The glycan flow was calculated by comparing the concentration of product glycan (Y) to the total concentration of product and substrate glycan (X + Y) as described below. An example of a glycomics analysis of an individual biosynthetic step in the mannose trimming pathways is shown below (Figure 2).
Figure 2.
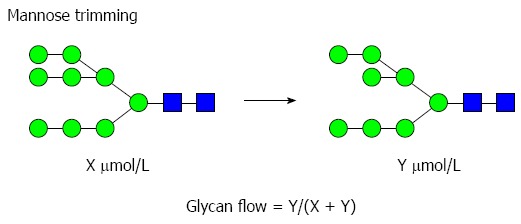
Example of a glycomics analysis of an individual biosynthetic step in the mannose trimming pathway.
The statistical significance of differences in the glycan flow was evaluated using the Student t-test. Glycan changes that yielded P values < 0.05 in this analysis were considered significant. The statistical analysis was carried out by Asma Bashir from UAE University.
RESULTS
DEN-induced foci of altered hepatocyte formation and GST-p expression
HCC was induced by DEN-2AAF, and HCC induction in the rat model was verified by assessing serum AFP (data not shown) as reported previously[28]. The histological examination was carried out to assess HCC induction. Hepatic nodules were evident only in animals treated with DEN-2AAF, but not in normal rats (Figure 3). These nodules represent the classical foci of altered hepatocytes and are made up of big and irregularly shaped hepatocytes with large hyperchromatic nuclei. As induced GST-p is normally considered an early biomarker of hepatocarcinogenesis, GST-p foci larger than 15 cells were examined using a color image processor. The GST-p positive foci were significantly increased in animals treated with DEN-2AAF. Histological examination provided evidence of successful tumor development in DEN-induced HCC rats.
Figure 3.
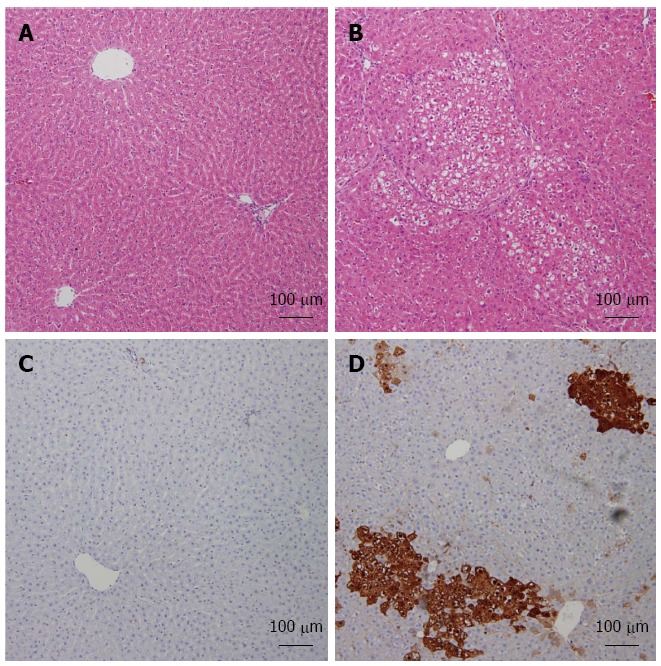
Diethyl nitrosamine-induced increase of foci in altered hepatocytes and induction of GST-p expression in liver. Representative images of Hematoxylin and Eosin-stained liver sections from both studied groups: Normal (A), hepatocellular carcinoma (HCC) (B). Immunohistochemistry analysis of both normal (C) and HCC (D) livers labeled with GST-p are shown.
Glycan analysis
The Ezose Science proprietary GlycanMap® methodology (Figure 1A) was utilized to quantify N-linked glycans. This technique was previously developed by Nishimura, Furukawa and Miura[29-31]. Repeatability of the assay was evaluated using a standard human serum sample. Five aliquots of the standard were analyzed in parallel with the individual rat liver lysate samples and used to evaluate repeatability. Coefficients of variation (CVs) for individual glycans ranged from 7.5% to 24.8%, with a pooled CV of 14.8%. An overview of the N-glycan profile in rat liver is shown in Figure 1B. In total, 29 glycans were detected in our study, Figure 3A represents the overall profile of N-glycans analyzed. The glycan structure and the code used are shown in Figure 4.
Figure 4.
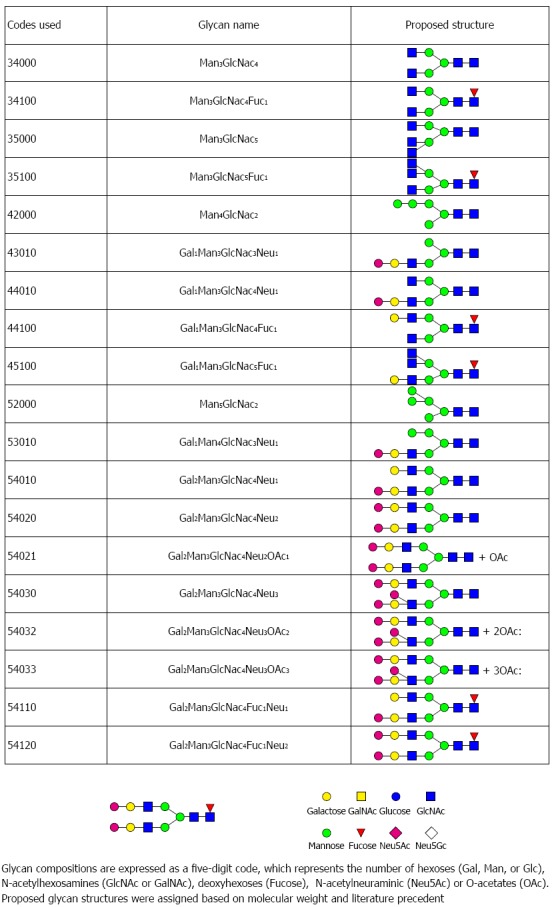
This figure lists the glycan codes and proposed structures for all 29 glycans detected in this study.
HCC is associated with glycan level changes
Twenty nine glycans were detected and the changes in these glycans were analyzed. Glycans; Glc1Man9GlcNAc2 (code: 102000), Gal2Man3GlcNac4Fuc1Neu1 (code: 54110), Man4GlcNac2 (code: 42000), Gal2Man3GlcNac4Neu3OAc3 (code: 54033), and Man3GlcNac5Fuc1, (code: 35000) showed significant changes in rat HCC liver tissues when compared with normal liver tissues. Four were increased and one was decreased in HCC rats compared to normal rats (Figure 5).
Figure 5.
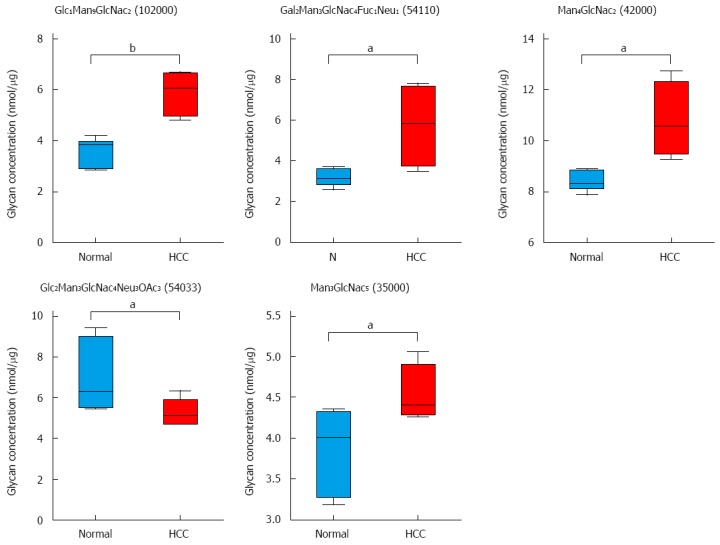
Hepatocellular carcinoma-related alterations in glycans in tumor-bearing animals. Five glycans changed in tumor-bearing rats compared to normal rats. A combined scatter and box-and-whisker plot is shown for each glycan, along with an indication of the statistical significance (aP < 0.05, bP < 0.01 vs control, n = 7).
Differential effects of HCC on glycans
The glycans detected can be classified into three categories including high mannose (simple mannose chains), hybrid (with fucosylation and sialylation) and complex (with complex branching structures) (Figure 6A). High mannose glycans, with the exception of Man8 (Man6, Man7, Man9 and Man10) were elevated (P < 0.05) in tumor-bearing rats compared to normal rats (Figure 6B). A non-significant decrease in fucosylated and sialylated glycans in HCC liver tissues was seen. However, a significant increase (P < 0.05) in sialyl-Lewis glycans (i.e., with fucose, galactose and sialyl groups) and O-acetylated glycans was seen in HCC liver tissues.
Figure 6.
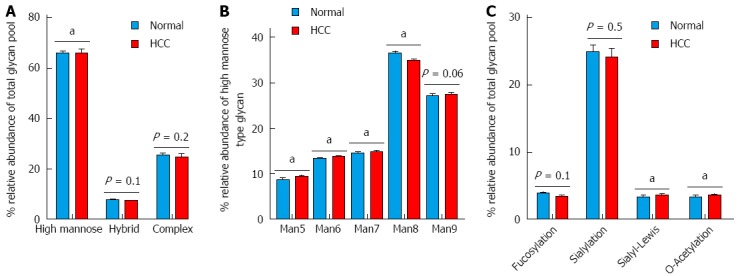
Differential expression of N-glycans structures and determinants. A: N-Glycan structures i.e., high mannose, hybrid and complex; B: Distribution of the different types of high mannose structures (Man5 to Man9); C: Distribution of terminal glycan determinants: fucosylation, sialylation, sialyl-Lewis and O-acetylation. The groups were compared by t-test and aP < 0.05 vs control n = 7.
Glycan fluctuations are correlated with their subcellular localization
Analysis of core glycans based on the subcellular compartment/s in which they are synthesized, was useful. Glycans synthesized in ER, cis- and trans-Golgi were all increased in HCC tissues compared to normal liver tissues. Although not all were significantly increased, the overall trend was a higher level in HCC tissues. The levels of glycans synthesized in medial-Golgi did not appear to follow a trend (Figure 7C).
Figure 7.
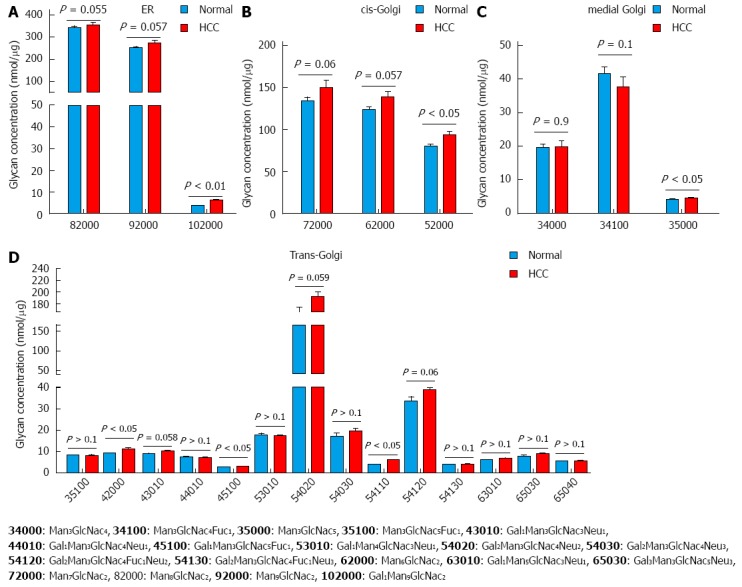
Analysis of glycan biosynthesis based on the compartments in which they are synthesized. The glycans are synthesized in ER-Golgi by a chain of enzymes. Glycans synthesized. A: ER; B: Cis-Golgi; C: Medial-Golgi; D: Trans-Golgi. The groups were compared by t-test, P < 0.05, P < 0.01 vs control, n = 7.
HCC alters the glycan flow
To correlate the alterations in N-glycans with the enzymes involved in the biosynthetic pathway, the glycan flow was analyzed based on the ratio of adjacent glycans in the synthetic pathway. N-Glycans are synthesized from Glc3Man9 in ER and Golgi via long chain glycosyltransferases (GT) and glycosylhydrolases (GH), and glycan flow is directly related to enzyme activity involved in these reactions[32]. In our study, the glycan flow was significantly decreased from Glc1Man9 to Man9 (P < 0.005) (Figure 8A) explaining the increase in glycan Glc1Man9 levels (Figure 5A). A decrease (P = 0.057) in glycan flow in other mannose trimming reactions in the ER (from Man9 to Man8) was also observed. Glycan flow in cis-Golgi increased in HCC rats compared to normal rats with Man8 to Man7 flow increasing significantly (P < 0.05) (Figure 8B). The complex glycans synthesized in trans-Golgi showed an increase in flow from Man3GlcNac4Fuc1 to Gal2Man3GlcNac4Fuc1Neu2 in trans-Golgi (Figure 8D). This explains the increase in Gal2Man3GlcNac4Fuc1Neu1 (Figure 5) in HCC liver tissues, which is an intermediate glycan in the reaction of Man3GlcNac4Fuc1 to Gal2Man3GlcNac4Fuc1Neu2.
Figure 8.
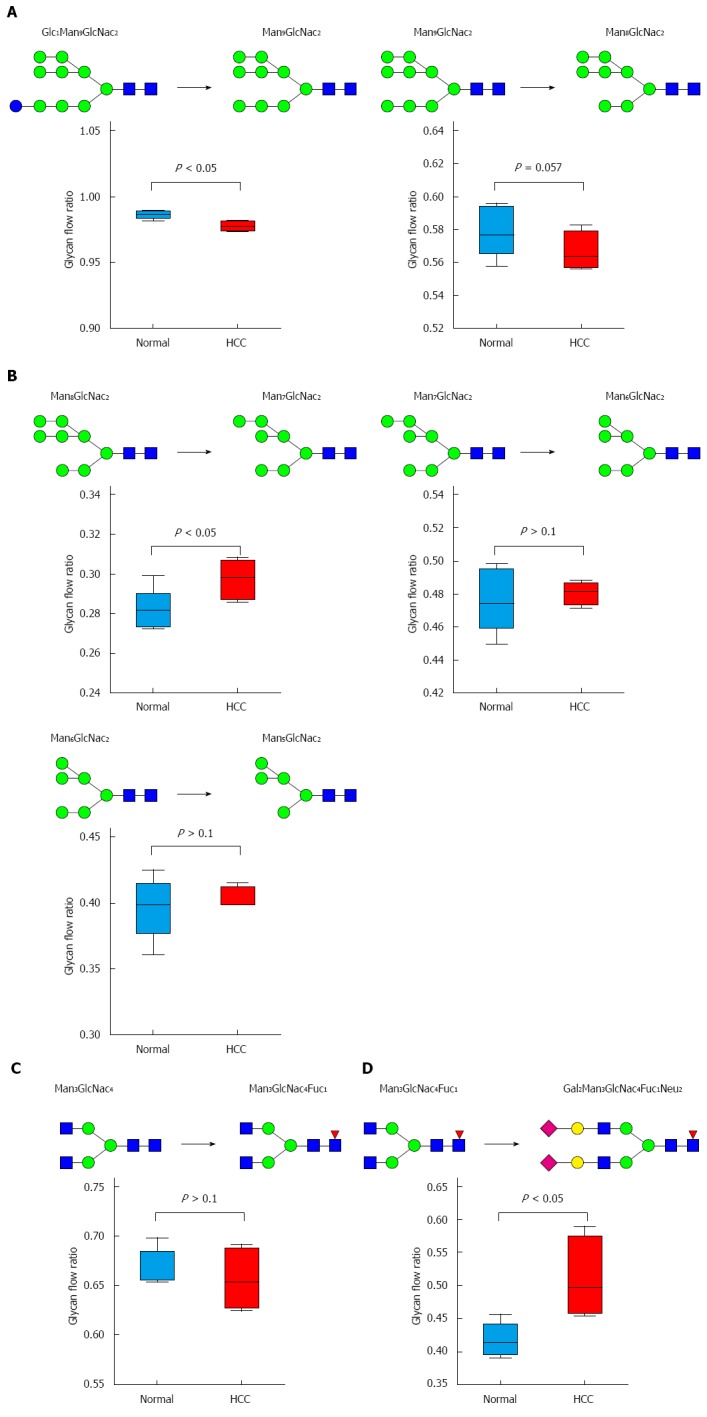
Glycan flow. Changes in glycan flow in the N-glycan biosynthesis pathway. The ratio was compared by t-test and P value < 0.05 was considered significant. A: ER; B: Cis-Golgi; C: Medial-Golgi; D: Trans-Golgi.
DISCUSSION
Glycosylation is sensitive to microenvironment alterations and thus is involved directly in metastatic diseases such as cancer. These changes in glycosylation lead to the formation of tumor-specific glycans which are now believed to play an important role as biomarkers[33,34]. The relatively high stability of glycans compared to other molecules (RNA and proteins) along with the development of new techniques such as MALDI-TOF and ESI profile have revolutionized the field of glycan research. Interestingly, aberrant glycosylation has been shown to develop before any changes in apoptosis and cell differentiation associated with cancer[35].
High mannose glycans have been shown to be prevalent in HCC liver tissues compared to normal liver tissues. One high mannose, Glc1Man9GlcNAc2, was significantly elevated in HCC rats compared to normal rats in the present study (Figure 5). A similar increase in high mannose glycans was reported in other cancers[36,37]. High mannose glycans have also been shown to increase in invasive and non-invasive breast cancer cells compared to normal epithelial cells[36,37]. Another study showed up-regulation of high mannose oligosaccharides on cell surface glycoproteins in cancer cells compared to normal cells[38]. N-linked glycans are constructed by a series of glycosyl transferases both in the ER and Golgi stacks. The chain starts with high mannose type glycans and then sequential mannose removal and addition of N-acetylglucosamine and glucose residues leads to the formation of complex and hybrid glycans. Thus, high mannose glycans may be increased (accumulated) if either the mannose trimming enzyme is altered or downstream processing events are terminated or abberated. ER mannosidase I (ERManI), an ER-based mannosidase, is responsible for a step-wise trimming of Man residues transforming Man9 to Man5[39]. ERManI expression has been shown to be aberrant in liver tissues of HCC patients and in hepatoma cells lines[40], which explains the increase in glycan Glc1Man9GlcNAc2 (code: 102000) in the present study (Figure 5). Studies have shown that ERManI knockdown leads to accumulation of Man9 and Gal1Man9, and a decrease in Man5 and Man6 compared to normal tissues[39].
In liver cancer, induction of both α-1,6- and α-1,3-linked fucosylation has been reported as the most prominent serum N-glycosylation change associated with that disease[41-43]. In the present investigation, fucosylated or sialylated glycans did not increase in HCC liver tissues compared to normal liver tissues, consistent with a study performed on human liver tissues of HCC patients[25]. However, there was an increase in the relative abundance of sialyl Lewis glycans (fucose, galactose and NeuAc groups) in DEN-induced HCC. The sialyl-Lewis glycans are commonly abberated TAC in cancers[44,45]. One such glycan, Gal2Man3GlcNac4Fuc1Neu1 (code: 54110) was increased in HCC rats compared to normal rats (Figure 5). TACs are expressed both by tumor and host cells and are involved in the key pathophysiological processes during the various steps of tumor progression, including tumor growth, cell migration, invasion, metastasis, angiogenesis, and evasion of innate immunity[8,46-50]. TACs are studied extensively due to their potential as specific tumor biomarkers.
Branched glycans are synthesized by adding antennae branching structures by glycotransferases (GnT) such as N-acetylglucosaminyltransferase V (GnT-V). GnT-V is the key enzyme that catalyzes the formation of 1,6 N-acetylglucosamine (GlcNAc) and adding it to a common core structure of Man3GlcNAc2 in the medial-Golgi apparatus. One product of this reaction is Man3GlcNac5 (code: 35000), which was observed to be significantly increased in HCC rats compared to normal rats (Figure 5). Man3GlcNac5 is a branched glycan synthesized from Man3GlcNAc2 by GnTs. Overexpression of GnTs is associated with metastasis and their implication in HCC have already been reported[51-53].
Sialylation, the addition of sialic acid moieties, is an important modification of N-glycans that may alter the physical properties of molecules in the plasma membrane and may regulate immune cell function, as well as serve as specific ligands for certain toxins and lectins[54,55]. Sialic acids e.g., N-acetylneuraminic acid (Neu5Ac) are capping structures on the glycans and may be further modified by various functional groups including acetyl, lactyl, methyl, sulfate, or phosphate groups. One such glycan, Gal2Man3GlcNac4Neu3OAc3 (54033) with three o-acetyl groups was shown to be significantly decreased in HCC rats compared to normal rats (Figure 4). Changes in the O-acetyl group are seen as irrelevant in terms of their role in disease prognosis. Recently, however, O-acetlylated Neu5Ac has been shown to contribute to drug resistance in acute lymphoblastic leukemia cells and its removal made the cells vulnerable to cytotoxic drugs[56].
Subcellular localization and flow changes in N-linked glycans showed that the processing starts in the ER by sequential removal of glucose and mannose from Glc3Man9GlcNAc2 which continues in the Golgi complex. Mannose trimming is carried out by a series of mannosidases and extension is performed in Golgi by glycotransferases producing the complex-type and hybrid type. This study showed that changes in N-linked glycans followed a pattern depending on subcellular localization (Figure 7), which can be explained by the changes seen in glycan flow in the N-glycan biosynthesis pathway (Figure 8). An increase in high mannose glycans (Figure 6A) correlates with decreased glycan flow, showing significant ER stress in HCC liver tissues. An increase in sialyl-Lewis compared to fucosylated glycans (Figure 6C) can also be explained by the increased glycan flow in trans-Golgi which is the normal location for processing complex/hybrid glycans. The glycan levels may not give a clear indication of the disturbance in the synthesis pathway, but it is clearly depicted by glycan flow. The glycan flow clearly depicts the changes in enzymes involved in N-glycan biosynthesis.
In conclusion, we conducted a detailed analysis of N-glycans in HCC tissues compared to normal liver tissues. Five glycans were significantly altered. Together, the changes in glycan flow and subcellular localization provide an explanation for the increase in high mannose glycans and siayl Lewis glycans reported in HCC tissues.
ACKNOWLEDGMENTS
Authors are grateful to Aktham Awad and Sayel Daoud (Tawam Hospital) for histology, Alaa Hamza (UAE University) for helping with the animals. Authors are also indebted to Youssef Abdalla (Groves High, MI, United States) for his technical assistance.
COMMENTS
Background
Glycosylation is a crucial post-transcriptional modification of many proteins. Protein glycosylation (N- and O- glycosylation) may result from disruption of the biosynthetic pathway such as the ER-Golgi chain and is commonly disrupted in various diseases such as cancer. Some tumor-associated carbohydrate (TAC) antigens are already in clinical use for the diagnosis and monitoring of cancers. Glycomics is a fast-growing recent technology that has been utilized as a new potential tool for cancer prognosis and treatment. The lack of reliable diagnostic biomarkers has turned hepatocellular carcinoma (HCC) into the prime target of many glycomic-based analyses. Numerous investigations have shown significant changes in total N-glycan levels, including Alpha Fetoprotein (AFP), in the serum of HCC patients, thereby demonstrating the potential of glycans and the glycome profile as efficient biomarkers of HCC. As the only known biomarker used for HCC, levels of AFP remain unchanged during the onset of HCC, which makes its application as a differential diagnosis marker of other liver diseases unreliable. Although fucosylated glycans have been shown to increase in HCC patients compared to patients with chronic liver diseases, no such increase was reported in the liver tissues of HCC patients when compared with normal tissues. Therefore, glycan analyses of cancer tissues have become a priority.
Research frontiers
In an attempt to utilize recent technology such as glycomics, the present study focuses on the analysis of the N-glycan changes in chemically-induced HCC liver tissues. Tissue glycan profiles were analyzed using the Ezose GlycanMap® platform to identify changes in the N-glycans of liver tissues in a HCC rodent model.
Innovations and breakthroughs
The fact that defective glycosylation normally develops prior to any changes in apoptosis and cell differentiation associated with cancer has driven us to study how glycans are synthesized in HCC livers. This study showed a significant increase in the high mannose, Glc1Man9GlcNAc2, in HCC rats compared to normal rats. As the increase of such high mannose glycans may result from alterations in the mannose trimming enzyme, ER mannosidase I (ERManI), ERManI was also studied here. Similar to the livers of HCC patients, ERManI expression was shown to be aberrant as reflected in the increase in glycan Glc1Man9GlcNAc2 in the present study. TACs are studied extensively due to their potential as specific tumor biomarkers. Thus, as in different types of cancers, the sialyl-Lewis glycans (fucose, galactose and NeuAc groups) are commonly abberated, the present study showed an increase in Gal2Man3GlcNac4Fuc1Neu1 in HCC rats compared to normal rats. This study also showed a significant decrease in acylated Gal2Man3GlcNac4Neu3OAc3 in HCC rats compared to normal rats. Recently, O-acetlylated Neu5Ac (sialic acid) has been shown to contribute to drug resistance in acute lymphoblastic leukemia cells and its removal made the cells vulnerable to cytotoxic drugs. This investigation also showed a patterned change in N-linked glycans that depended on their subcellular localization. The increased high mannose glycans (decreased glycan flow) reflects a major ER stress in HCC tissues.
Applications
The present detailed glycomic analysis of the N-glycans in HCC tissues compared to normal liver tissues has led to a better understanding of how the changes in glycan flow and subcellular localization can provide an explanation of the increase in high mannose glycans and siayl Lewis glycans reported in HCC tissues. This may potentially lead to the identification of new and more reliable biomarkers of HCC.
Terminology
Liver cancer was chemically induced in rats. Liver tissues were collected from both cancer-induced and normal animals for further studies. An HCC-specific antigen (GST-p) was targeted with an antibody (Anti- GST-p) to confirm tumor formation. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis was performed and mass spectra were analyzed using Ezose’s proprietary bioinformatics programs. Glycan amounts were compared between the different animal groups.
Peer-review
These findings provide new index to diagnose the HCC, which has potential applications in clinic. This study did good work and used modern techniques to illustrate their conclusion. The authors have identified specific glycans from HCC tissue.
Footnotes
Supported by National Research Foundation Grant No. UIRCA 2012-21832 for A. Amin.
Ethics approval: The study was reviewed and approved by UAE University No.1185/10.
Institutional animal care and use committee: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the UAE University (Ethics approval No.1185/10).
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at (a.amin@uaeu.ac.ae).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 27, 2014
First decision: December 26, 2014
Article in press: February 13, 2015
P- Reviewer: Al-Gayyar MMH, Tsunedomi R, Yin YJ S- Editor: Qi Y L- Editor: Webster JR E- Editor: Ma S
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Cazet A, Julien S, Bobowski M, Krzewinski-Recchi MA, Harduin-Lepers A, Groux-Degroote S, Delannoy P. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr Res. 2010;345:1377–1383. doi: 10.1016/j.carres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 5.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin Chem. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 6.Korekane H, Hasegawa T, Matsumoto A, Kinoshita N, Miyoshi E, Taniguchi N. Development of an antibody-lectin enzyme immunoassay for fucosylated α-fetoprotein. Biochim Biophys Acta. 2012;1820:1405–1411. doi: 10.1016/j.bbagen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 8.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomme B, Van Steenkiste C, Callewaert N, Van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J Hepatol. 2009;50:592–603. doi: 10.1016/j.jhep.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Meany DL, Chan DW. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin Proteomics. 2011;8:7. doi: 10.1186/1559-0275-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callewaert N, Van Vlierberghe H, Van Hecke A, Laroy W, Delanghe J, Contreras R. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–434. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 13.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Vanderschaeghe D, Laroy W, Sablon E, Halfon P, Van Hecke A, Delanghe J, Callewaert N. GlycoFibroTest is a highly performant liver fibrosis biomarker derived from DNA sequencer-based serum protein glycomics. Mol Cell Proteomics. 2009;8:986–994. doi: 10.1074/mcp.M800470-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gui HL, Gao CF, Wang H, Liu XE, Xie Q, Dewaele S, Wang L, Zhuang H, Contreras R, Libert C, et al. Altered serum N-glycomics in chronic hepatitis B patients. Liver Int. 2010;30:259–267. doi: 10.1111/j.1478-3231.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, Wang L, Zhuang H, Callewaert N, Libert C, et al. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426–1435. doi: 10.1002/hep.21855. [DOI] [PubMed] [Google Scholar]
- 17.Vanhooren V, Liu XE, Franceschi C, Gao CF, Libert C, Contreras R, Chen C. N-glycan profiles as tools in diagnosis of hepatocellular carcinoma and prediction of healthy human ageing. Mech Ageing Dev. 2009;130:92–97. doi: 10.1016/j.mad.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Miyahara K, Nouso K, Miyake Y, Nakamura S, Obi S, Amano M, Hirose K, Nishimura S, Yamamoto K. Serum glycan as a prognostic marker in patients with advanced hepatocellular carcinoma treated with sorafenib. Hepatology. 2014;59:355–356. doi: 10.1002/hep.26531. [DOI] [PubMed] [Google Scholar]
- 19.Tang Z, Varghese RS, Bekesova S, Loffredo CA, Hamid MA, Kyselova Z, Mechref Y, Novotny MV, Goldman R, Ressom HW. Identification of N-glycan serum markers associated with hepatocellular carcinoma from mass spectrometry data. J Proteome Res. 2010;9:104–112. doi: 10.1021/pr900397n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saffroy R, Pham P, Reffas M, Takka M, Lemoine A, Debuire B. New perspectives and strategy research biomarkers for hepatocellular carcinoma. Clin Chem Lab Med. 2007;45:1169–1179. doi: 10.1515/CCLM.2007.262. [DOI] [PubMed] [Google Scholar]
- 21.Karvountzis GG, Redeker AG. Relation of alpha-fetoprotein in acute hepatitis to severity and prognosis. Ann Intern Med. 1974;80:156–160. doi: 10.7326/0003-4819-80-2-156. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi N. Toward cancer biomarker discovery using the glycomics approach. Proteomics. 2008;8:3205–3208. doi: 10.1002/pmic.200890056. [DOI] [PubMed] [Google Scholar]
- 23.Noda K, Miyoshi E, Uozumi N, Yanagidani S, Ikeda Y, Gao C, Suzuki K, Yoshihara H, Yoshikawa K, Kawano K, et al. Gene expression of alpha1-6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of alpha-fetoprotein. Hepatology. 1998;28:944–952. doi: 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- 24.Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802–1806. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 25.Mehta A, Norton P, Liang H, Comunale MA, Wang M, Rodemich-Betesh L, Koszycki A, Noda K, Miyoshi E, Block T. Increased levels of tetra-antennary N-linked glycan but not core fucosylation are associated with hepatocellular carcinoma tissue. Cancer Epidemiol Biomarkers Prev. 2012;21:925–933. doi: 10.1158/1055-9965.EPI-11-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blomme B, Heindryckx F, Stassen JM, Geerts A, Colle I, Van Vlierberghe H. Serum protein N-glycan alterations of diethylnitrosamine-induced hepatocellular carcinoma mice and their evolution after inhibition of the placental growth factor. Mol Cell Biochem. 2013;372:199–210. doi: 10.1007/s11010-012-1461-1. [DOI] [PubMed] [Google Scholar]
- 27.Espandiari P, Robertson LW, Srinivasan C, Glauert HP. Comparison of different initiation protocols in the resistant hepatocyte model. Toxicology. 2005;206:373–381. doi: 10.1016/j.tox.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron: a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura S, Niikura K, Kurogochi M, Matsushita T, Fumoto M, Hinou H, Kamitani R, Nakagawa H, Deguchi K, Miura N, et al. High-throughput protein glycomics: combined use of chemoselective glycoblotting and MALDI-TOF/TOF mass spectrometry. Angew Chem Int Ed Engl. 2004;44:91–96. doi: 10.1002/anie.200461685. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa J, Shinohara Y, Kuramoto H, Miura Y, Shimaoka H, Kurogochi M, Nakano M, Nishimura S. Comprehensive approach to structural and functional glycomics based on chemoselective glycoblotting and sequential tag conversion. Anal Chem. 2008;80:1094–1101. doi: 10.1021/ac702124d. [DOI] [PubMed] [Google Scholar]
- 31.Miura Y, Shinohara Y, Furukawa J, Nagahori N, Nishimura S. Rapid and simple solid-phase esterification of sialic acid residues for quantitative glycomics by mass spectrometry. Chemistry. 2007;13:4797–4804. doi: 10.1002/chem.200601872. [DOI] [PubMed] [Google Scholar]
- 32.Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. doi: 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]
- 34.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 35.Konska G, Guerry M, Caldefie-Chezet F, De Latour M, Guillot J. Study of the expression of Tn antigen in different types of human breast cancer cells using VVA-B4 lectin. Oncol Rep. 2006;15:305–310. doi: 10.3892/or.15.2.305. [DOI] [PubMed] [Google Scholar]
- 36.Goetz JA, Mechref Y, Kang P, Jeng MH, Novotny MV. Glycomic profiling of invasive and non-invasive breast cancer cells. Glycoconj J. 2009;26:117–131. doi: 10.1007/s10719-008-9170-4. [DOI] [PubMed] [Google Scholar]
- 37.de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB. High-mannose glycans are elevated during breast cancer progression. Mol Cell Proteomics. 2011;10:M110.002717. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johns TG, Mellman I, Cartwright GA, Ritter G, Old LJ, Burgess AW, Scott AM. The antitumor monoclonal antibody 806 recognizes a high-mannose form of the EGF receptor that reaches the cell surface when cells over-express the receptor. FASEB J. 2005;19:780–782. doi: 10.1096/fj.04-1766fje. [DOI] [PubMed] [Google Scholar]
- 39.Avezov E, Frenkel Z, Ehrlich M, Herscovics A, Lederkremer GZ. Endoplasmic reticulum (ER) mannosidase I is compartmentalized and required for N-glycan trimming to Man5-6GlcNAc2 in glycoprotein ER-associated degradation. Mol Biol Cell. 2008;19:216–225. doi: 10.1091/mbc.E07-05-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan S, Wang S, Utama B, Huang L, Blok N, Estes MK, Moremen KW, Sifers RN. Golgi localization of ERManI defines spatial separation of the mammalian glycoprotein quality control system. Mol Biol Cell. 2011;22:2810–2822. doi: 10.1091/mbc.E11-02-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comunale MA, Wang M, Hafner J, Krakover J, Rodemich L, Kopenhaver B, Long RE, Junaidi O, Bisceglie AM, Block TM, et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J Proteome Res. 2009;8:595–602. doi: 10.1021/pr800752c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, Evans AA, Hann HW, Block TM, Mehta AS. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;5:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. The alpha1-6-fucosyltransferase gene and its biological significance. Biochim Biophys Acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- 44.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 45.Pochechueva T, Jacob F, Fedier A, Heinzelmann-Schwarz V. Tumor-associated glycans and their role in gynecological cancers: accelerating translational research by novel high-throughput approaches. Metabolites. 2012;2:913–939. doi: 10.3390/metabo2040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danussi C, Coslovi A, Campa C, Mucignat MT, Spessotto P, Uggeri F, Paoletti S, Colombatti A. A newly generated functional antibody identifies Tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology. 2009;19:1056–1067. doi: 10.1093/glycob/cwp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011;113:236–247. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 49.Powlesland AS, Hitchen PG, Parry S, Graham SA, Barrio MM, Elola MT, Mordoh J, Dell A, Drickamer K, Taylor ME. Targeted glycoproteomic identification of cancer cell glycosylation. Glycobiology. 2009;19:899–909. doi: 10.1093/glycob/cwp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 51.Jin XL, Liu HB, Zhang Y, Wang BS, Chen HL. Alteration in N -acetylglucosaminyltransferase activities and glycan structure in tissue and bile glycoproteins from extrahepatic bile duct carcinoma. Glycoconj J. 2004;20:399–406. doi: 10.1023/B:GLYC.0000033996.86169.bb. [DOI] [PubMed] [Google Scholar]
- 52.D’Arrigo A, Belluco C, Ambrosi A, Digito M, Esposito G, Bertola A, Fabris M, Nofrate V, Mammano E, Leon A, et al. Metastatic transcriptional pattern revealed by gene expression profiling in primary colorectal carcinoma. Int J Cancer. 2005;115:256–262. doi: 10.1002/ijc.20883. [DOI] [PubMed] [Google Scholar]
- 53.Mizuochi T, Nishimura R, Derappe C, Taniguchi T, Hamamoto T, Mochizuki M, Kobata A. Structures of the asparagine-linked sugar chains of human chorionic gonadotropin produced in choriocarcinoma. Appearance of triantennary sugar chains and unique biantennary sugar chains. J Biol Chem. 1983;258:14126–14129. [PubMed] [Google Scholar]
- 54.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parameswaran R, Lim M, Arutyunyan A, Abdel-Azim H, Hurtz C, Lau K, Müschen M, Yu RK, von Itzstein M, Heisterkamp N, et al. O-acetylated N-acetylneuraminic acid as a novel target for therapy in human pre-B acute lymphoblastic leukemia. J Exp Med. 2013;210:805–819. doi: 10.1084/jem.20121482. [DOI] [PMC free article] [PubMed] [Google Scholar]


