Table 1.
Potential sites amenable for optimization and ELISA results of marinopyrroles.
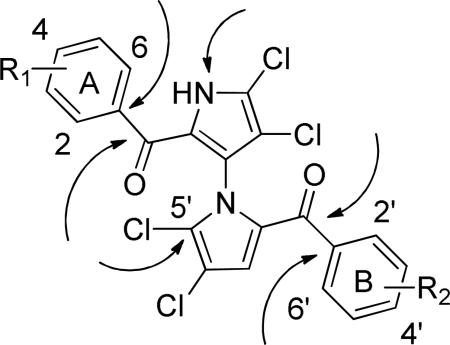
| ||||
|---|---|---|---|---|
| ID | R1 | R2 | Mcl-1/Bima | Bcl-xL/Bima |
| 1b | 2-OH | 2′-OH | 8.9 ± 1.0 | 16.4 ± 3.3 |
| 23c | 2-OH-4-CF3 | 2′-OH-4′-CF3 | 8.1 ± 0.9 | 9.7 ± 1.3 |
| 24 | 2-OH-5-Cl-4-Me | 2′-OH-5′-Cl-4′-Me | 2.6 ± 0.6 | 2.5 ± 0.4 |
| 25 | 2-OH-4-C=CH | 2′-OH-4′-C=CH | 3.9 ± 0.2 | 5.6 ± 0.5 |
| 26 | 2-OH-4-CH=CH2 | 2′-OH-4′-CH=CH2 | 3.7 ± 0.5 | 3.5 ± 0.7 |
| 27 | 2-OH-4-Et | 2′-OH-4′-Et | 2.1 ± 0.5 | 3.9 ± 1.3 |
| 28c | 2-OH-4-OSO2CF3 | 2′-OH-4′-OSO2CF3 | 1.0 ± 0.3 | 2.1 ± 0.7 |
| 29c | 2-OH-4-OH | 2′-OH-4′-OH | 39.5 ± 6.2 | >50 |
| 30c | 2-OH-5-Cl-4-OH | 2′-OH-5′-Cl-4′-OH | 10.7 ± 0.2 | >50 |
| 31d | 2-OH-4-SCH2CO2Et | 2′-OH-4′-SCH2CO2Et | 1.8 ± 0.3 | 1.2 ± 0.2 |
| 32d | 2-OH-4-SCH2Ph | 2′-OH-4′-SCH2Ph | 0.7 ± 0.2 | 0.6 ± 0.2 |
| 33d | 2-OH-4-SCH2(p-MeOPh) | 2′-OH-4′-SCH2(p-MeOPh) | 0.7 ± 0.1 | 0.6 ± 0.1 |
| 34d | 2-OH-4-SCH2CO2H | 2′-OH-4′-SCH2CO2H | 6.1 ± 1.3 | >100 |
| 35e | 2-OH-4-PO(OH)2 | 2′-OH-4′-PO(OH)2 | 10.9 ± 3.1 | 27.3 ± 7.2 |
| 36 |
|
|
7.8 ± 1.5 | >100 |
| 37 |
|
|
1.6 ± 0.6 | 14.0 ± 4.7 |
| 38 |
|
|
5.2 ± 0.8 | >50 |
| 39 |
|
|
3.3 ± 0.9 | 1.6 ± 0.3 |
| 40 |
|
|
1.5 ± 0.2 | 0.8 ± 0.2 |
| 41 |
|
|
1.4 ± 0.5 | 3.8 ± 1.3 |
| 42 |
|
|
0.6 ± 0.3 | 0.5 ± 0.1 |
| 43 |
|
|
18.4 ± 0.3 | >100 |
| 44 |
|
|
5.1 ± 0.4 | 8.1 ± 2.5 |
| 45 |
|
|
16.5 ± 1.9 | >50 |
| 46f | 2-OMe | 2′-OMe-4′-Cl | 8.0 ± 1.6 | 9.5 ± 2.2 |
| 47f | 2-OH | 2′-OH-3′-Cl | 4.1 ± 1.4 | 10.1 ± 2.2 |
| 48f | 2-OH | 2′-OH-5′-Cl | 3.9 ± 1.1 | 18.3 ± 3.0 |
| 49f | 2-OH | 2′-OH-4′-Cl | 6.5 ± 1.3 | 9.2 ± 2.3 |
| 50g | 2-OH | 2′-OH-5′-F | 8.9 ± 0.9 | 13.3 ± 3.3 |
| 51g | 2-OH | 2′-OH-4′-F | 9.6 ± 0.4 | 21.3 ± 5.6 |
| 52g | 2-OH | 2′-OH-6′-F | 13.1 ± 0.3 | 43.7 ± 10.0 |
| 53 | 2-OMe | 2′-OMe and N-Meh | 15.5 ± 3.3 | 64.9 ± 15.5 |
| 54 | 2-OH | 2′-OH and N-Meh | > 100 | 7.9 ± 1.8 |
| ABT-263 | 4.3 ± 0.4 nM | |||
IC50 in μM (average ± SEM, n ≥ 3) unless specified
Activity as disruptors of Mcl-1 and Bc1-xl reported previously and here for SAR discussion [13]
Chemistry, anti-MRSA activity was reported previously [12]
Chemistry and activity as disruptors of Mcl-1 and Bcl-xL reported previously and here for SAR discussion [15]
Chemistry and activity as disruptors of Mcl-1 and Bcl-xL reported previously and here for SAR discussion [13]
Chemistry and anti-MRSA activity were reported previously [5]
Chemistry and anti-MRSA activity were reported previously [14]
N-Methyl analogue.
