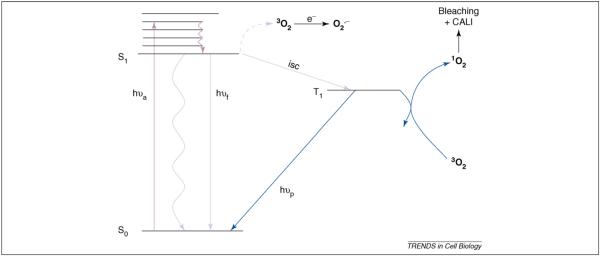
Figure 3.
ROS production in terms of simplified energy-level diagram. Excitation (denoted by absorption of a photon, hυa) occurs to one of several higher vibronic states followed by rapid internal conversion (compact squiggly arrow) resulting in the molecule dropping to the lowest excited singlet state (S1) in which it exists for several nanoseconds. From this state, several fates are possible: the excited chromophore can emit (denoted by emission of a photon, hυf), transfer its energy to the solvent without emission (extended squiggly arrow), stimulate electron transfer from 3O2 (depicted as the broken line from S1 to 3O2) to produce superoxide anion (O2•−) or undergo intersystem crossing (isc) to the triplet manifold. (Note, to simplify the diagram, generation of 1O2 from the chromophore single state is not depicted.) The triplet state is long lived and, for this reason, much photochemistry proceeds from it, including ROS production; emission from the triplet state is called phosphorescence (denoted by emission of a photon, hυp).