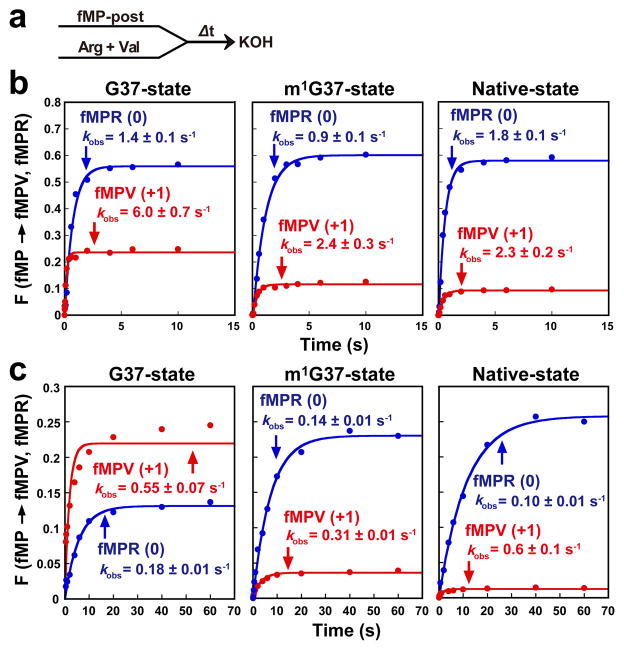
Figure 3. Peptide bond formation by a post-translocation complex with GGG or UGG tRNAPro stalled at the P-site.
a. Kinetic scheme for measurement of kobs of peptide bond formation upon rapid mixing of excess ternary complexes of tRNAArg and tRNAVal (molar ratio of 1:1) with a stalled post-initiation complex carrying fMP-tRNAPro/GGG or fMP-tRNAPro/UGG in the P-site. The coding mRNA sequence was AUG-CCC-CGU-U. b. Measurement of the fractional conversion F of total fMP molecules to the in-frame fMPR product (blue) and to the +1-frame fMPV product (red) over time for G37- (left), m1G37- (middle), and native-state (right) tRNAPro/GGG, showing the rate constant of conversion (kobs) for each. Yields of the respective tripeptides did not change when the molar ratio of tRNAArg to tRNAVal was varied from 1:5 to 5:1 or when each incoming tRNA ternary complex was added separately to a limiting amount of the post-translocation complex. c. Similar analysis conducted with tRNAPro/UGG (anticodon presented 5′ to 3′) in the P-site of the post-translocation complex. Error range of the curve fitting is denoted.