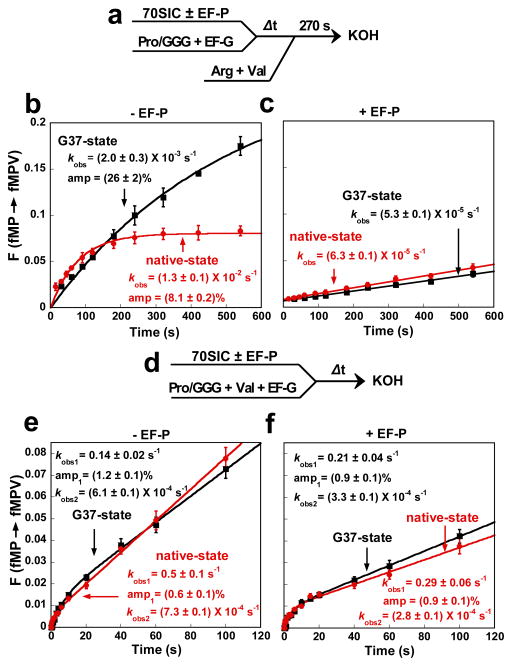
Figure 4. Formation of +1FS errors by GGG tRNAPro at the 2nd codon.
a. The kinetic scheme to measure the rate of +1FS formation by fMP-tRNAPro/GGG stalled at the P-site of a post-translocation complex on the template AUG-CCC-CGU-U. A 70SIC was mixed with Pro-tRNAPro/GGG and EF-G to form a stalled post-translocation complex, which was sampled over time by mixing with excess ternary complexes of tRNAArg and tRNAVal (molar ratio of 1:1). After 270 sec of peptide synthesis, each secondary reaction was quenched and the peptides analyzed. b. Time course of the fractional conversion F from total fMP molecules to fMPV molecules, showing the kinetics of +1-frameshifting by the G37- (black) and native-state (red) of tRNAPro/GGG at 20 °C. c. Analysis as in b but in the presence of EF-P (10 μM). d. The kinetic scheme to measure the rate of +1FS formation by fMP-tRNAPro/GGG when a 70SIC was rapidly mixed with ternary complexes of tRNAPro and tRNAVal in the presence of EF-G. This onestep reaction format precluded stalling of the intermediate fMP-post-translocation complex. e. Biphasic kinetics were observed for +1FS by both the G37- (black) and native-state (red) tRNAPro/GGG. f. Analysis as in e but in the presence of EF-P (10 μM). The kobs and amplitude of each shift are indicated, each as the average of at least three independent measurements. Error bars denote SD.