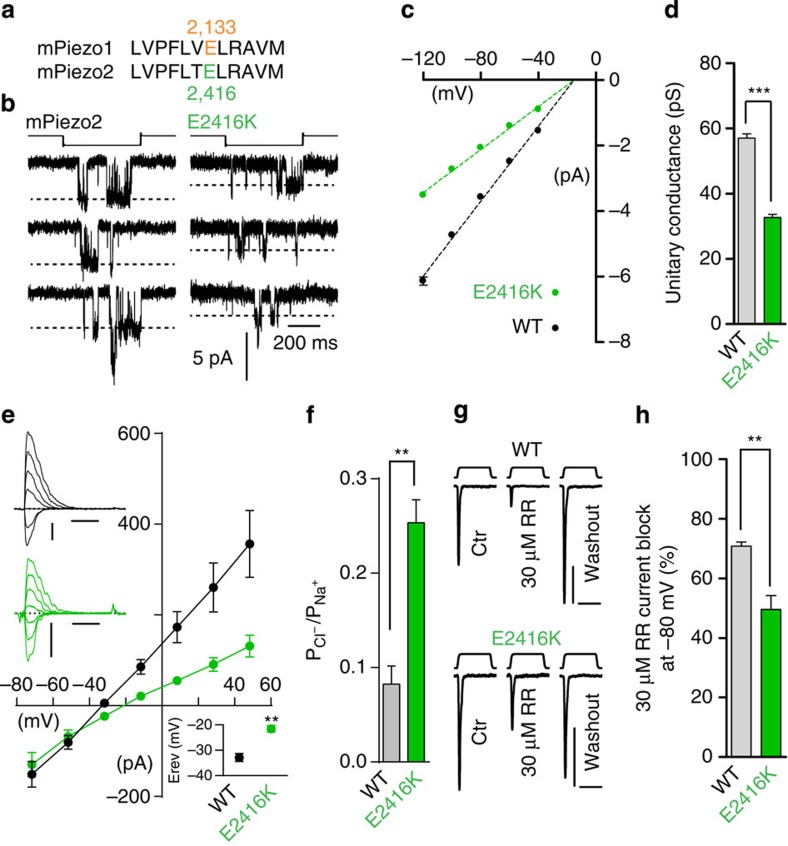
Figure 6. Lysine substitution of E2416 alters pore properties of mPiezo2 channels.
(a) Conservation of mPiezo1 E2133 at position 2,416 on mPiezo2 protein. (b) Representative (from nine and six experimental replicates) stretch-activated channel openings at −80 mV from cells transfected with mPiezo2 WT and E2416K. Stimulation intensities are −15 and −20 mm Hg, respectively. (c) Average I–V relationships of stretch-activated single channels in mPiezo2 WT and E2416K transfected cells (n=9 and 6, respectively; mean±s.e.m.). Single-channel amplitude was determined as the amplitude difference in Gaussian fits of full-trace histograms. (d) Single-channel conductance calculated from the slope of linear regression line of individual cell single-channel I–V relationships (mean±s.e.m.; Mann–Whitney test, ***P<0.001). (e) Average I–V relationships of MA currents recorded from mPiezo2 WT and E2416K expressing cells with 150 mM NaCl-based intracellular solution and 30 mM NaCl extracellular solution. Top left inset: typical recording traces for WT (black) and E2416K (green) from −71.7 to +48.3 mV, Δ20 mV. Scale bars, 100 pA, 50 ms. Probe stimulation displacements are 9 and 8 μm, respectively. Bottom right inset: average reversal potential (mean±s.e.m.; n=6 and 5, respectively; Mann–Whitney test **P<0.01). (f) PCl/PNa permeability ratios of MA currents from cell transfected with mPiezo2 WT, and E2416K (mean±s.e.m.; n=6 and 5, respectively; Mann–Whitney test **P<0.01). (g) Representative (from five experimental replicates) MA current traces from mPiezo2 WT and E2416K transfected cells at −80 mV before, during or after application of 30 μM RR. Each trace is an average of two to four trials. Probe stimulation displacements are 5 and 7 μm, respectively. Scale bars, 100 pA, 100 ms. (h) Average block of MA currents at −80 mV by 30 μM RR in mPiezo2 WT and E2416K transfected cells (n=5 for each; mean±s.e.m.; Mann–Whitney test, **P<0.01). Experiments shown in b, c and d were performed in cell-attached configuration with Na+ as the only permeating cation in the recording pipette.