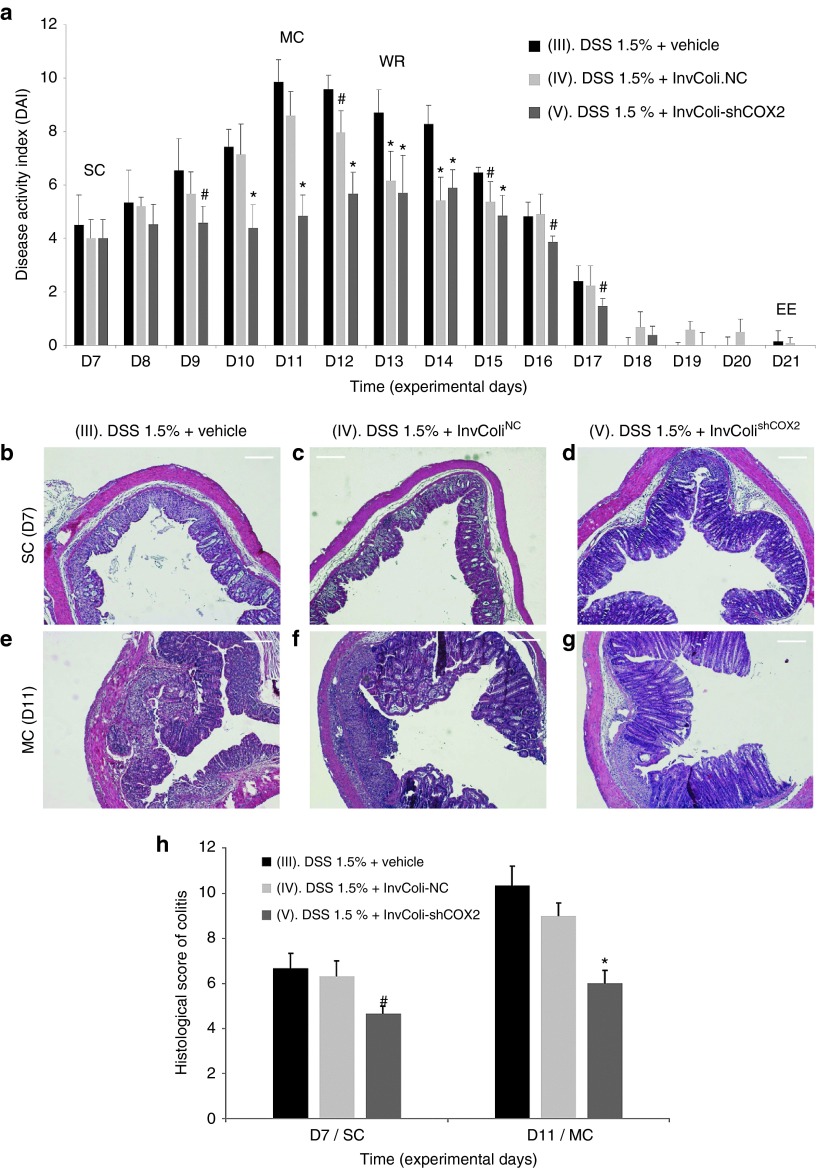
Figure 2.
Effect of the InvColishCOX2 strategy on DSS-induced colitis. (a) The biological effect of InvColi/RNAi-mediated COX-2 silencing was evaluated considering the disease activity index (DAI) of colitis, calculated by the combined score of weight loss, stool consistency, and bleeding (see also Supplementary Figures S2b and S4). All parameters were scored from experimental day 7 (D7) to experimental day 21 (D21). Experimental mice groups: (III) “DSS 1.5% + vehicle”; (IV) “DSS 1.5% + InvColiNC”; (V) “DSS 1.5% + InvColishCOX2.” Vehicle, LB medium; EE, end of experiment; MC, maximum colitis; SC, start colitis; WR, weight recovery. Data represent the mean + SD of independent measurements; per group: n = 12 (D7), n = 9 (D8–D11), n = 6 (D12–D13), n = 3 (D14–D21). *P < 0.01; #P < 0.05. (b,g) A histological analysis was carried out on FFPE colon specimens from group III, IV, and V. Colon specimens were collected on day 7 (D7, start colitis, SC) and day 11 (D11, maximum colitis, MC). Scale bar = 200 µm; magnification ×50. (h) Quantification of histological differences between experimental mice group III, IV, and V at times D7 and D11, based on the scoring parameters shown in Supplementary Figure S2c. Data represent the mean + SEM of independent measurements (n = 3 per group). *P < 0.01. #P < 0.05.