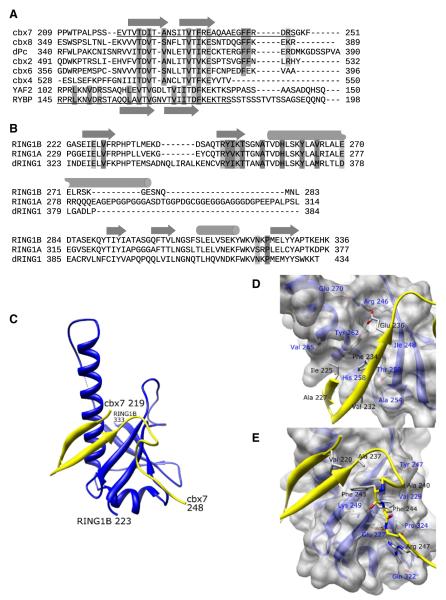
Figure 1. C-RING1B/cbx7 cbox Structure.
(A) ClustalW sequence alignment of the cbox domains from all five human and Drosophila Pc proteins and the region of the human RYBP sequence known to associate with C-RING1B (Garcia et al., 1999). The underlined sequences represent the residues used in the crystallization.
(B) The C-terminal domains from human RING1A, RING1B, and Drosophila RING1 proteins. Secondary structures are indicated as arrows (beta sheet) and cylinders (helix). Residues that mediate hydrophobic and polar interactions are dark and light shaded, respectively.
(C) Structure of the C-RING1B cbx7 cbox complex. The RING1B is blue, cbx7 is yellow. Termini are labeled. The dotted line indicates RING1B residues 285 and 288 which could not be modeled because of weak density.
(D and E) A closeup view of the (D) beta sheet and (E) the loop interactions. Cbx7 residues 219–224 are deleted in (D) for clarity. C-RING1B residues are labeled in blue, cbx7 residues are labeled in black.