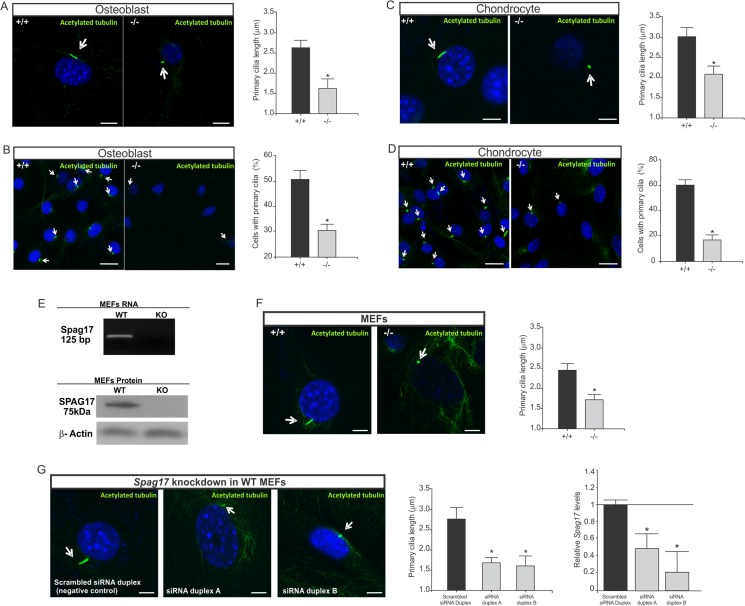
Fig 5. Primary cilia are altered in Spag17-deficient osteoblast, chondrocyte and MEFs cells.
Cells were stained with anti-acetylated α-tubulin to visualize primary cilia and DAPI as a nuclei marker. (A) Primary cilia length from osteoblast cells. Scale bars, 5 μm. (B) Percentage of osteoblast cells expressing primary cilia. Scale bars, 20 μm (C) Primary cilia length from chondrocyte cells. Scale bars, 5 μm. (D) Percentage of chondrocyte cells expressing primary cilia. Scale bars, 20 μm. Mouse embryonic fibroblasts (MEFs) were isolated from embryos at E12.5. (E) Detection of Spag17 mRNA and protein in MEFs. RT-PCR products were generated by primers from exon 4 and 5. The knockout mice have a deletion of the entire exon 5. Detection of SPAG17 protein by western blot using an antibody against C-terminal domain. (F) After serum-starvation to promote cilia growth, primary cilia were visualized in the MEFs with acetylated tubulin antibody, and DAPI as a nuclei marker. Primary cilia from Spag17-mutant MEFs were significantly shorter than wild-type. Scale bars, 5 μm. (G) Spag17 knockdown in WT MEFs cells after treatment with Spag17 siRNA duplex reproduced the shorter primary cilia phenotype. Scale bars, 5 μm. Arrows indicate primary cilia. (+/+), wild-type, (-/-); Spag17-mutant mice. Data are presented as means ± SEM. * Indicates statistically significant differences, p< 0.05.